
Root systems are responsible for all water and nutrient uptake by the plant and they provide the physical anchoring and support of the plant structure. Each plant and crop species has its own “personality” and growth habits, and root systems have unique characteristics among plants species.
In general plant root systems constitute 30-50% of the total plant dry matter. When post-harvest plant residues are incorporated into the soil, the root systems provide a significant contribution to that plant material and final carbon (C) contributions to the soil.
The first thing a seed develops in the germination process is a primary root that grows downward into the soil. We often refer to this as the “stinger” root that extends from a germinating seed. New cells are formed at the tip of the primary root as it extends downward into the soil forming a “thimble-shaped” cluster of cells called a root cap (Figure 1). The root cap serves as a type of shield that helps the root penetrate the soil matrix and protect the developing root tissue. As the root grows downward into the soil the root cap cells are sloughed off creating a slimy surface that helps lubricate the root as it extends deeper.
The growing point (apical meristem) for the developing root is just behind the root cap and this is the zone of new cell formation that facilitates root growth and replaces the cells that are sloughed off as the root grows through the soil. The new cells elongate and serve to extend the roots into the soil (Figure 1).
The most active parts of the plant root system for mineral nutrient and water uptake are in the tiny root hairs that are formed in zone behind the apical meristem. Root hairs are only formed in the relatively new and freshly developed root tissue. The root hairs are extremely small, tender, and physiologically active. Root hairs are often referred to as “feeder roots” due to their high-level of activity in securing water and nutrients from the soil for the growing plant. In the process of transplanting, it is important to protect the feeder roots and promote their health to ensure rapid adaptation to the new soil environment.
Young plants have the capacity to develop basic aboveground tissue to perform sufficient photosynthesis for establishment and growth due to the plant’s ability to take up mineral nutrients and water from the soil from the root system. Sometimes it can appear that plants are not growing rapidly while the young crop is investing energy and resources into root system development, which is the foundation for the subsequent plant growth and development.
The depth of the roots will vary according to the soil physical conditions and effective soil depth, soil fertility and salinity management, plant-available water, and of course the natural rooting characteristics of the plant.
In general, there are two basic types of plant root systems. Broadleaf plants (dicotyledonous) and coniferous plants (gymnosperms) commonly have a taproot system the extends downward through the soil developing root branches from the primary root stem (Figure 2).
Grass plants and their relatives (monocotyledonous plants) produce fibrous root systems that branch extensively and radiate out into the soil from the plant base (Figure 2).
In general, taproots tend to be deeper with extensive branching from the primary root, develop woody tissue on older roots, and generally long-lived. In contrast, fibrous roots tend to be smaller, short-lived, with less branching. As roots age, they become more important in conducting nutrients and water to the growing points of the plant, both above and belowground. In all cases, the young and freshly developed root hairs (feeder roots) are the primary zone of water and mineral nutrient uptake.
As root systems age, the older roots will die, and new root tissue is formed. As dead roots are sloughed off, the discarded tissue is attacked by naturally occurring, beneficial soil organisms (bacteria, fungi, protozoa, and worms) the release mineral nutrients and produce soil organic matter. Turnover of root tissue is an extremely important aspect of plant contributions to soil carbon (C) and organic matter.
We do not see the plant root system on a regular basis and we cannot watch root hair development. But it is good to be conscious of root system development since all mineral nutrient, water uptake, and structural support is provided through the roots. So, it is good to review and understand root structure and function as we work to manage crop plants for optimum growth and development.
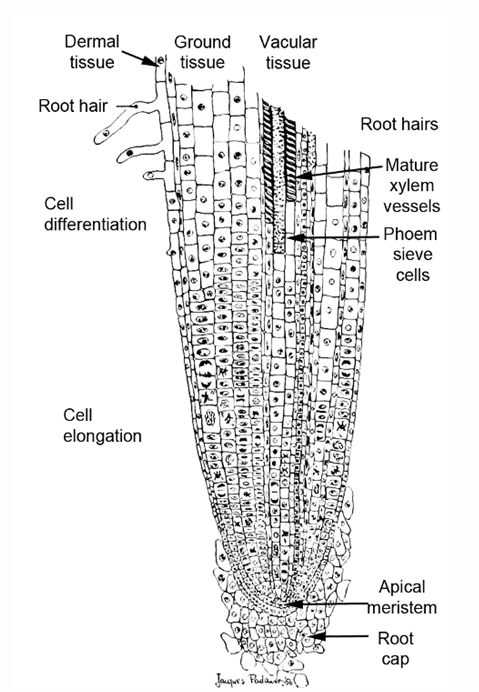
Figure 1. Basic root tip anatomy.
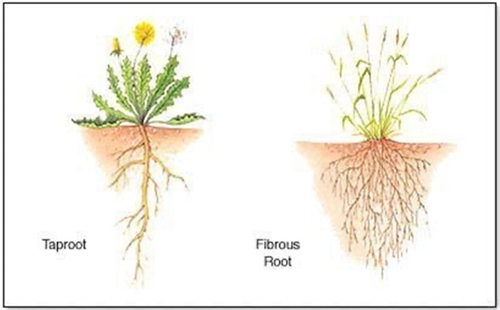
Figure 2. Examples of taproot and fibrous root systems.
This study was conducted at the Yuma Valley Agricultural Center. The soil was a silty clay loam (7-56-37 sand-silt-clay, pH 7.2, O.M. 0.7%). Spinach ‘Meerkat’ was seeded, then sprinkler-irrigated to germinate seed Jan 13, 2025 on beds with 84 in. between bed centers and containing 30 lines of seed per bed. All irrigation water was supplied by sprinkler irrigation. Treatments were replicated four times in a randomized complete block design. Replicate plots consisted of 15 ft lengths of bed separated by 3 ft lengths of nontreated bed. Treatments were applied with a CO2 backpack sprayer that delivered 50 gal/acre at 40 psi to flat-fan nozzles.

Downy mildew (caused by Peronospora farinosa f. sp. spinaciae)was first observed in plots on Mar 5 and final reading was taken on March 6 and March 7, 2025. Spray date for each treatments are listed in excel file with the results.
Disease severity was recorded by determining the percentage of infected leaves present within three 1-ft2areas within each of the four replicate plots per treatment. The number of spinach leaves in a 1-ft2area of bed was approximately 144. The percentage were then changed to 1-10scale, with 1 being 10% infection and 10 being 100% infection.
The data (found in the accompanying Excel file) illustrate the degree of disease reduction obtained by applications of the various tested fungicides. Products that provided most effective control against the disease include Orondis ultra, Zampro, Stargus, Cevya, Eject .Please see table for other treatments with significant disease suppression/control. No phytotoxicity was observed in any of the treatments in this trial.
At the 2023 Southwest Ag Summit Field Demo a couple of weeks ago, many of the latest technologies were demonstrated in the field. Most were related to pest control. Several of the technologies demonstrated or on display at the event are brand new to the Yuma, AZ area. The new technologies presented included an autonomous orchard sprayer (Fig. 1a), wide span (4 and 6 row) automated weeders (Fig. 1b, Fig. 1c), steam applicators for pre-plant weed control (Fig. 1d) and post-emergent weed control/crop desiccation (Fig. 1e) and a camera guided cultivator (Fig. 1f). This trend towards developing wider and more productive machine machines is indicative of a maturing industry. It will be interesting to watch these machines evolve further and become integrated in our cropping systems.

Fig. 1. New pest control technologies demonstrated/on display at the 2023 Southwest Ag Summit Field Demo included a) mini GUSS1 autonomous orchard sprayer, b) K.U.L.T.i - Select automated weeder, c) FarmWise’s Vulcan automated weeder, d) UC Davis/UofA band-steam applicator, e) X-Steam-inator’s steam applicator and f) Mantis Ag Technology’s camera-guided cultivator. (Photo credits Fig. 1d: Mazin Saber, University of Arizona)
____________________
[1] Reference to a product or company is for specific information only and does not endorse or recommend that product or company to the exclusion of others that may be suitable.
Common Purslane (Portulaca oleracea) is a very widespread weed in desert southwest and a problem for vegetable production. At the same time, it is one of the most nutritious leafy vegetables. It has been reported that Common Purslane contains five times higher omega-3 fatty acids than spinach which are important for human growth, development, also prevent of numerous cardiovascular diseases, and maintain a healthy immune system. It also contains vitamins A, B and C and dietary minerals. Common Purslane is consumed in Mexico (Verdolaga), Europe, Asia, and the Mediterranean Region.
Portulaca oleracea can be confused with Horse Purslane but they belong to different families. Common Purslane is in the Portulacaceae family and Horse Purslane (Trianthema portulacastrum) is in the Aizoaceae or Ice plant family1.
One of the IPM strategies for Purslane control in lettuce production is germinating it during ground preparation then kill it with chemicals or tillage. Timing is important because Purslane grows fast and it’s a very prolific seed producer. These seeds can germinate in 12 hours after receiving moisture in August and September. They can also germinate in January and February but will take 3-7 days to germinate at that time. The stems are very succulent and unless they are completely killed and desiccated, they can reroot at the nodes. Tillage that does not completely desiccate the plants can spread rather than eliminate this weed3.
Some herbicides that will kill this weed during ground preparation are Gramoxone, Aim, and ET and systemic (glyphosate) herbicides. Results can range from depending upon weed size, rate and adjuvant used at the time of application. The contact herbicides can produce almost 100% control when the Purslane is less than 2 inches in diameter and less than 50% control when larger than this. Recently we conducted an evaluation in which 2oz of ET with a silicone spreader controlled 100% of the population. According to some researchers control with some contact herbicides can drop from excellent to poor in 3 - 5 days3.
We are evaluating application methods and incorporation timings of Prefar (bensulide) at Yuma Ag Center the and the product continues showing a good performance in controlling Purslane as can be seen in the images below.

Figure 1. Evaluation of bensulide herbicide applications incorporated with sprinkler irrigation.
Results of pheromone and sticky trap catches can be viewed here.
Corn earworm: CEW moth counts down in all traps over the last month; about average for December.
Beet armyworm: Moth trap counts decreased in all areas in the last 2 weeks but appear to remain active in some areas, and average for this time of the year.
Cabbage looper: Moths increased in the past 2 weeks, and average for this time of the season.
Diamondback moth: Adults increased in several locations last, particularly in the Yuma Valley most traps. Below average for December.
Whitefly: Adult movement remains low in all areas, consistent with previous years
Thrips: Thrips adult movement continues to decline, overall activity below average for December.
Aphids: Winged aphids still actively moving but declined movement in the last 2 weeks. About average for December.
Leafminers: Adult activity down in most locations, below average for this time of season.