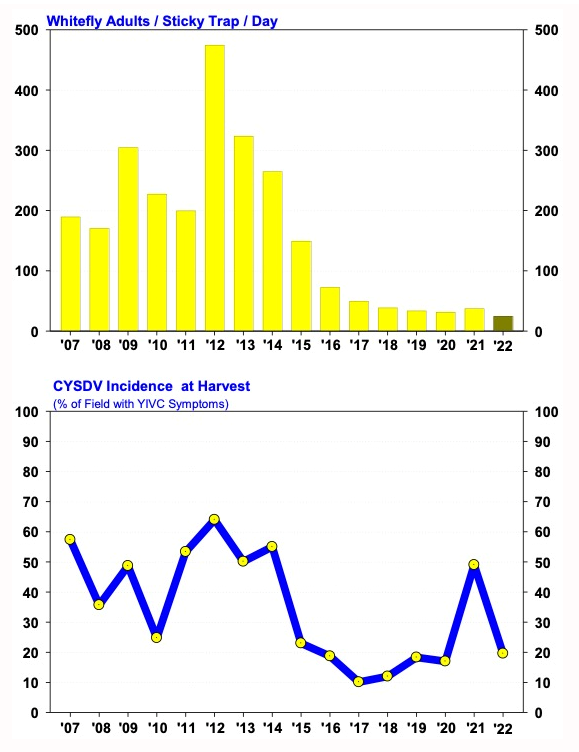
Nov 29, 2023
Soil Health: Cation Exchange
Soils provide the foundation of crop production systems and there are several chemical properties worthy of our attention (Parikh and James, 2012). In consideration of the three main categories (physics, chemistry, and biology) describing a soil system in relation to soil health, soil chemical properties represent an extremely important aspect of healthy soil function (Figure 1).
Figure 1. Soil health factors and chemical properties.
The solid phase of soils is composed of both mineral and organic components. The mineral portion of the soil consists of sand, silt, and clay fractions. In this article, the focus is on the mineral portion of the soil system and the basis of soil cation exchange capacity (CEC).
The relative proportions of sand, silt, and clay fractions define the soil texture (Figure 3). Soil texture is probably the most fundamental characteristic of a soil since it affects many of the important physical, biological, and chemical processes in a soil. Despite any management practice that is used in a field, the soil texture changes very little over time.
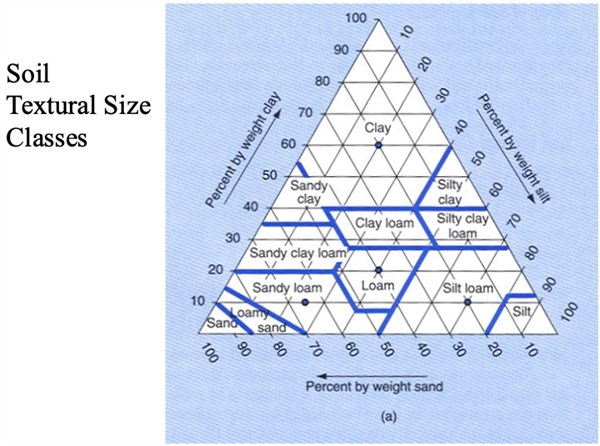
Figure 2. Soil textural triangle.
One aspect of soil texture that is very important in relation to soil chemical properties is the clay or colloidal fraction, particles less than 0.002 mm or the less than 2 µm (micron) in diameter. This is important because of the extremely small size of the particles which renders a very large amount of surface area among the clay particles or the soil colloidal fraction (Figure 3).
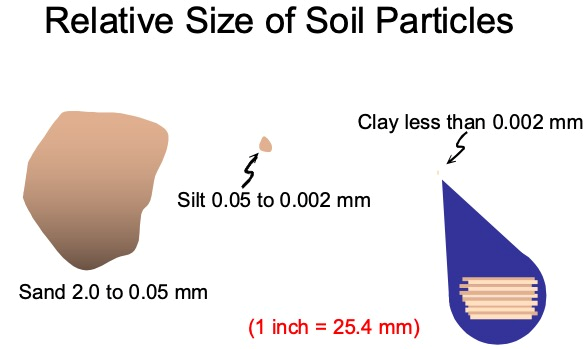
Figure 3. Relative size of soil particles.
In the natural process of soil formation and both chemical and physical weathering the secondary clay minerals, layer silicates, are formed. Fine-grained micas, chlorite, and vermiculite are formed through the basic processes of weathering of primary aluminosilicate minerals. Kaolinite and oxides of iron and aluminum are products of much more intense or longer duration weathering.
Soil composition is a direct reflection of the materials from which they are made. Parent material for the mineral fraction of soils are normally the primary aluminosilicate minerals. As the name implies, these minerals are made from aluminum (Al), silicon (Si), and oxygen (O). Collectively, these elements account for approximately 82 % of the earth’s crust by weight. On a mass basis, O and Si are extremely important and are dominate components in mineral soils (Table 1).
|
Element
|
% by Weight
|
Element
|
% by Weight
|
|
O (oxygen)
|
46.5
|
Ca (calcium)
|
3.6
|
|
Si (silicon)
|
27.6
|
Mg (magnesium)
|
2.1
|
|
Al (aluminum)
|
8.1
|
Na (sodium)
|
2.8
|
|
Fe (iron)
|
5.1
|
K (potassium)
|
2.6
|
Table 1. Average chemical composition of the earth's crust.
In soil minerals, both Si and Al are found as small, highly charged positive cations (Al3+ and Si4+), which coordinate with the larger and negatively charged O anion (O2-) to form silica tetrahedron and the aluminum octahedron. In both structures, the small central ions are surrounded by either four or six O2- or OH- ions in coordination which serve to "hide" the cations and expose the O or OH groups to the soil solution. On a volume basis, the larger anions dominate the soil minerals, both primary and secondary (Brady and Weil, 2008; Magdoff, and van Es, 2021).

Layer silicate clays
These important silicate clays are also known as phyllosilicates (Phyllon - leaf)
because of their leaf-like or plate like structure. These are made up of two kinds of
horizontal sheets. One dominated by silicon and other by aluminum and/or magnesium.
Silica tetrahedron: The basic building block for the silica-dominated sheet is a
unit composed of one silicon atom surrounded by four oxygen atoms. It is referred to as
the silica tetrahedron because of its four-sided configuration. It is based on an interlocking array or a series of these silica tetrahedra tied together horizontally by shared oxygen anions rendering a tetrahedral sheet (Figure 4).
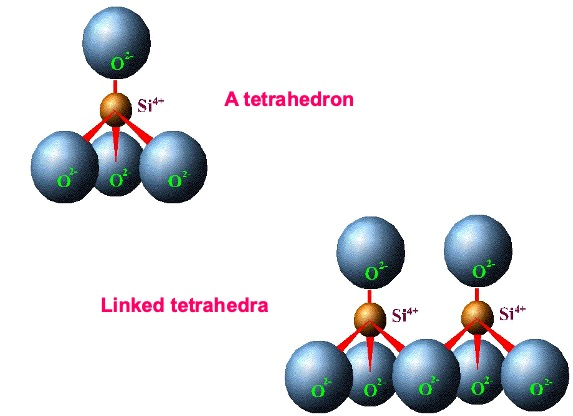
Figure 4. Layer silicate silicon tetrahedral structure.
Alumina octahedron: Aluminium and/or magnesium ions are the key cations surrounded by six oxygen atoms or a hydroxyl (OH-) group giving an eight-sided building block referred to as an octahedron. Numerous octahedra linked together horizontally comprise the octahedral sheet (Figure 5).

Figure 5. Layer silicate aluminum octahedral structure.
An aluminum-dominated sheet is known as a di-octahedral sheet, whereas one dominated by magnesium is called a tri-octahedral sheet.
The distinction is due to the two aluminum ions in a di-octahedral sheet that satisfy the same negative charge from surrounding oxygen and hydroxyls as three magnesium ions in a tri-octahedral sheet.
The tetrahedral and octahedral sheets are the fundamental structural units of silicate clays. These sheets are bound together within the crystals by shared oxygen atoms into different layers. The specific nature and combination of sheets in these layers vary from one type of clay to another and control the physical and chemical properties of each specific type of clay (Figure 6 and Table 2).
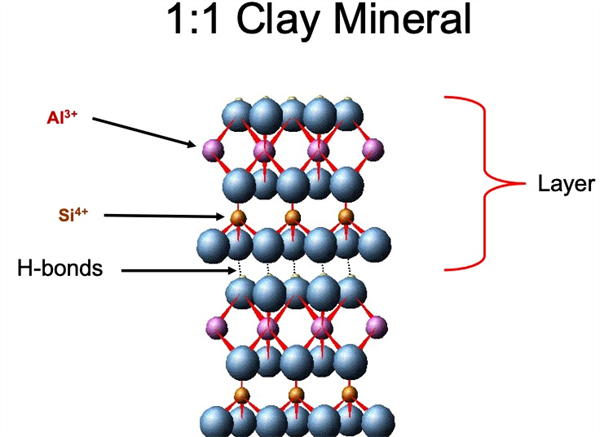
Figure 6. One to one clay mineral structure.
Isopmorphous Substitution
In some of the silica tetrahedra, Si4+ is replaced by Al3+ and the charge deficit (a net negative charge) is balanced by monovalent (single charge) or divalent (two charge unit) cations.
Similarly, in the octahedra, Al3+ may be replaced by Mg2+ or other divalent cations. This replacement also generates a net negative charge deficit that must be balanced by cations that are not an integral part of the octahedral or tetrahedral network.
In the cases of silica tetrahedra and aluminum octahedra, the substituting ions are of similar size. Thus, this exchange process is referred to as “isomorphous” substitution. Where “iso” means “same or similar” and “morphic or morphous” refers to the physical size and structure. This substitution refers to similar sized ion replacement but with different charge characteristics (Figures 7 and 8).

Figure 7. Isomorphic substitution in a clay mineral structure.
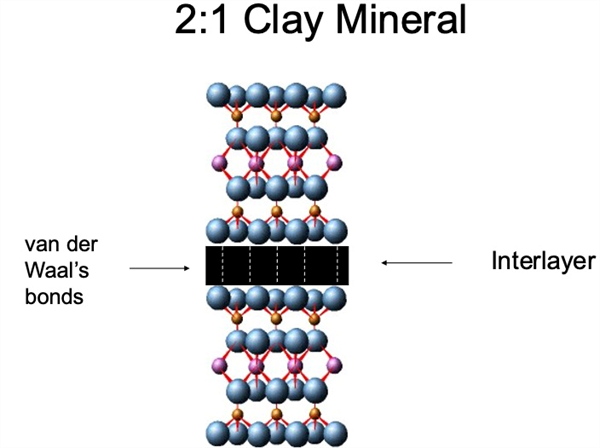
Figure 8. Two to one (2:1) clay mineral structure (two Si tetrahedrons and one
Al octahedral sheet in each layer) and the van der Waal’s bonds linking or
stacking the 2:1 layers.
Cation Exchange Capacity (CEC)
The result of this ionic substitution is a net negative charge on the surface of clay particles. The net negative on the soil clay or colloidal fraction provides a reactive surface of clay minerals that interact with cations (e.g., K+, Mg2+, Ca2+, Na+, etc.) in soil solution (Figure 8).
Thus, the greater the clay or colloidal fraction in the soil solid phase, the greater the reactive capacity the soil has. Since the net clay or colloid surface is negative, the reactions with the soil solution involve cations, both mineral and organic. Thus, this exchange phenomenon is referred to as “the cation exchange capacity, CEC” (Figure 8 and Table 2).
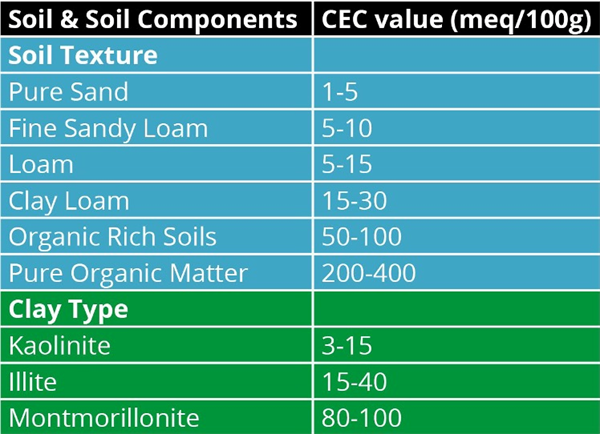
Table 2. Relationship between soil texture and clay type to the cation
exchange capacity (CEC, meq/100g).
The higher the soil CEC, the greater nutrient holding and exchange capacity of the soil. This aspect of a soil system also determines the water holding capacity to a very large extent because of the physical/chemical interaction between the soil colloid surface and water molecules (Figure 9).
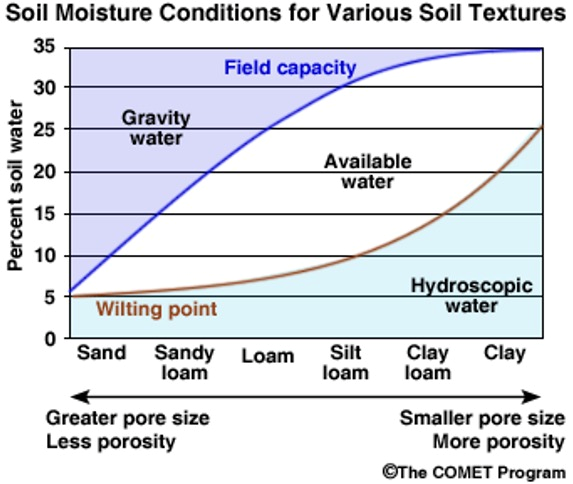
Figure 9. Soil-water holding capacity as a function of soil texture. Source: The
COMET program. University Corporation of atmospheric research.

Figure 10. A plant root hair extending into soil solution and interacting with
adjacent soil particles and cation exchange.
Healthy soil exhibits a full expression of the solid phase chemical capacity and function, including the cation exchange capacity (CEC), which largely defines the nutrient holding and exchange capacity of the soil (Figure 10). In this manner, a healthy soil system helps support healthy crops.
References:
Brady, N.C. and R.R. Weil. 2008. The Nature and Properties of Soils, 14th ed. Prentice Hall: Upper Saddle River, NJ.
Magdoff, F. and H. van Es, 2021. Soil Particles, Water and Air. In: Building Soils for Better Crops Sustainable Agriculture Research & Education Outreach Program. Chapter 5.
Parikh, S. J. and B.R. James. 2012. Soil: The Foundation of Agriculture. Nature Education Knowledge 3(10):2
https://www.nature.com/scitable/knowledge/library/soil-the-foundation-of-agriculture-84224268/#