
During the past 50-60 years, there have been some tremendous technological developments that have provided valuable tools enabling significant improvements in crop yields, quality, and production efficiencies. Some of the greatest of these developments have been in plant genetics.
In 1970, Dr. Norman Borlaug (Figure 1) received the Nobel Peace Prize for his work with CIMMYT (Centro Internacional de Mejoramiento de Maíz y Trigo, or the International Maize and Wheat Improvement Center) based in Ciudad Obregon, Sonora, Mexico. Borlaug and his CIMMYT colleagues were credited with the “Green Revolution” which was estimated to have saved at least 1B lives from starvation. Borlaug is the only agronomist to have received a Nobel Peace Prize.

Figure 1. Dr. Norman Borlaug, ca. 1963, Yaqui Valley, Sonora, Mexico.
Borlaug and his team accomplished two basic developments. First, using classical plant breeding methods, they bred dwarf wheat varieties that had a higher harvest index (a higher proportion of the total dry matter yield in the grain versus the vegetative parts of the plant). Second, they developed wheat varieties that were resistant to rust diseases, which had been devastating Mexican wheat crops for several decades. This CIMMYT program began in 1944 when Mexico was importing large amounts of wheat to support the population. Due to the success of this program, Mexico became a net exporter of wheat by 1963.
Borlaug and his colleagues transferred these new wheat varieties to southern Asia and between 1965 to 1970 wheat yields nearly doubled in Pakistan and India, greatly improving food security. These methods have been applied to other crops and other regions with great success (Borlaug, 2002).
In 1996 the first transgenic technologies were introduced in cotton and corn Bt varieties and utilized in crop production systems in the United States as result of molecular breeding programs. This incredible technology involved the insertion of genes from a common soil bacterium, Bacillus thuringiensis. These genes encode the production of insecticidal proteins, and thus, genetically transformed plants produce one or more toxins as they grow. The genes that have been inserted into these varieties produce toxins that are limited in activity almost exclusively to caterpillar pests (Lepidoptera family).
In the leafy green vegetable industry, new lettuce varieties have been developed over the past 40 years that offer the capacity for what is naturally a cool-season plant to be planted in the lower Colorado River Valley in August and September with average daily high temperatures of greater than 100°F. These are truly amazing developments from plant breeding programs.
The improved understanding in cellular-molecular biology and the details of DNA has led to new levels of capacity in crop improvement. This has led to the creation of molecular-markers, which are used in genomic-assisted plant breeding programs to identify genes linked to desired traits.
High-throughput phenotyping has provided the ability for plant geneticists to accurately identify and measure genetic traits. This provides the valuable capability of supplementing genomic information for precision breeding.
It is good to recognize and celebrate positive developments and achievements. Yet it is also important to recognize some major mistakes that have occurred in genetics and plant breeding.
In the early 20th century, a Russian agronomist Trofim Lysenko (Figure 2) was a proponent of Lamarckism, which rejected Mendelian genetics in favor of his own idiosyncratic, pseudoscientific ideas later referred to as Lysenkoism (Cashari and Marshak, 1965).
Lysenko became Director of the Russian Institute of Genetics of the Soviet Academy of Sciences in 1940. He used his political influence and power to suppress dissenting opinions and discredit, marginalize, and imprison his critics, elevating his anti-Mendelian theories to state-sanctioned doctrine, which was supported by Josef Stalin, the General Secretary of the Communist Party and dictator of the Soviet Union. Lysenko not only rejected Mendelian genetics but encouraged farmers to plant very high populations based on his belief in the “law of the life of species”, believing that plants from the same “class” will not compete for resources. That of course was a faulty line of thinking without foundation, and it created disastrous consequences.
Lysenko's ideas and practices in plant breeding and crop production practices contributed to the famines that killed millions of people in the Soviet Union. In addition, the adoption of his methods beginning in 1958 in the People's Republic of China had similarly disastrous results, contributing to the Great Chinese Famine of 1959 to 1961. Stalin favored people like Lysenko who was from a peasant family and was lacking in formal education and academic training and with no affiliations to the academic community. Despite Lysenko’s destructive record, he was promoted and awarded the Medal of Lenin eight times.
As a result of Lysenko’s unorthodox ideas and Stalin’s support of him, Soviet scientists who refused to renounce genetics were dismissed from their posts and left destitute. Many notable Russian scientists were imprisoned.
After tremendous amounts of human suffering due to Lysenko’s influence with the resultant crop failures and the death of Stalin in 1953, Lysenko began to lose favor in the Soviet Union. In 1965 he was finally removed from his position as the Director of the Institute of Genetics at the Academy of Sciences in the Soviet Union after the removal of Nikita Khrushchev as the first secretary of the Communist Party and Premier of the Soviet Union Nikita Khrushchev in 1964. Lysenko was ultimately disgraced in the late 1960s, but he did a lot of damage in 25 years.

Figure 2. Trofim Denesovich Lysenko, 1938.
The continued progress and genetic developments like those we have experienced in the past 60 years are not automatic and have required consistent and committed support in development of our basic understanding of plant genetics and biochemistry and the incorporation of that knowledge into well-directed plant breeding programs. Continued support and development basic science is essential for us to develop new tools with appropriate field applications.
We have many challenges in agriculture, and we need the benefit of good science and its application into our crop production systems to meet the needs of today and the future. We cannot afford a venture and descent into some form of Lysenkoism.
References:
Borlaug,N. E. 2002. "The green revolution revisited and the road ahead".Stockholm, Sweden. Nobelprize.org. https://www.nobelprize.org/uploads/2018/06/borlaug-lecture.pdf
Caspari,E. W.; Marshak, R. E. 1965. "The Rise and Fall of Lysenko". Science.149 (3681): 275–278. Bibcode:1965Sci... 149..275C.doi:10.1126/science.149.3681.275. PMID 17838094
To view this article as a PDF, click here and hit download.
Hi, I’m Chris, and I’m thrilled to be stepping into the role of extension associate for plant pathology through The University of Arizona Cooperative Extension in Yuma County. I recently earned my Ph.D. in plant pathology from Purdue University in Indiana where my research focused on soybean seedling disease caused by Fusarium and Pythium. There, I discovered and characterized some of the first genetic resources available for improving innate host resistance and genetic control to two major pathogens causing this disease in soybean across the Midwest.
I was originally born and raised in Phoenix, so coming back to Arizona and getting the chance to apply my education while helping the community I was shaped by is a dream come true. I have a passion for plant disease research, especially when it comes to exploring how plant-pathogen interactions and genetics can be used to develop practical, empirically based disease control strategies. Let’s face it, fungicide resistance continues to emerge, yesterday’s resistant varieties grow more vulnerable every season, and the battle against plant pathogens in our fields is ongoing. But I firmly believe that when the enemy evolves, so can we.
To that end I am proud to be establishing my research program in Yuma where I will remain dedicated to improving the agricultural community’s disease management options and tackling crop health challenges. I am based out of the Yuma Agricultural Center and will continue to run the plant health diagnostic clinic located there.
Please drop off or send disease samples for diagnosis to:
Yuma Plant Health Clinic
6425 W 8th Street
Yuma, AZ 85364
If you are shipping samples, please remember to include the USDA APHIS permit for moving plant samples.
You can contact me at:
Email: cdetranaltes@arizona.edu
Cell: 602-689-7328
Office: 928-782-5879
Since last season, there have been several new developments in commercial automated thinning and weeding technologies. Companies that focused primarily on weeding have developed new algorithms and/or improved precision so that the machines can now also be used for thinning lettuce. Significant enhancements have also been made to existing lettuce thinning machines including improved speed, durability and the ability to spot spray inter-row weeds. Arizona Cooperative Extension is in the process of organizing the Automated Thinner and Weeder Technologies Roundup, a field day where these technologies will be demonstrated operating in the field. NEW for this year will be that demos will be at the field scale level (> 1 acre) so attendees will have the opportunity to more fully evaluate machine performance and have time to visit with company representatives. The event will be held November 13th at the Yuma Agricultural Center in tandem with the Desert Difference Ag Connect Event. We would like to showcase as many innovative technologies as possible, so please contact me if you are interested in demoing your thinner/weeder or know someone that is. It’s an open invitation - private companies, university and government researchers are all welcome!

Fig. 1. Previous University of Arizona, Cooperative Extension AgTech Field Day Events
held at the Yuma Ag Center.
Recently, a sample of different species of sprangletop weed was sent to me by a PCA for identification purposes, highlighting the importance of accurately recognizing this troublesome group of grassy weeds. Sprangletop can appear similar across various species, but correct identification is crucial for effective management and herbicide selection. Sprangletop species belong to the genus Leptochloa and are generally summer annuals or short-lived perennials that thrive in wet or irrigated environments, often impacting specialty crops, orchards, and rangelands. The most common types you may encounter include Mexican sprangletop (Leptochloa fusca ssp. uninervia), green sprangletop (Leptochloa dubia), and bearded sprangletop (Leptochloa fascicularis).
Key Identification Features
Practical Tips
When inspecting sprangletop, focus on panicle shape and density, spikelet arrangement, and the presence or absence of awns. Note leaf blade length and texture as well as sheath color. Ligule characteristics—whether hairy, membranous, or jagged— can also aid identification. Correctly identifying the specific sprangletop type helps tailor weed control strategies, especially herbicide selection, since control efficacy can vary among species. For growers and PCAs encountering sprangletop challenges, collecting samples and seeking expert identification support is highly recommended, as illustrated by the recent field sample I received. Understanding these distinctions enhances integrated weed management efforts and supports cleaner, more productive crops.

| Mexican sprangletop (Leptochloa fusca ssp. uninervia) |
Green sprangletop (Leptochloa dubia) |

Bearded sprangletop (Leptochloa fascicularis) Source: https://weedid.missouri.edu/weedinfo.cfm?weed_id=473
References:
Flea beetles can be serious pests of vegetable crops. Unmanaged populations can lead to substantial crop losses and cosmetic damage, particularly to leafy vegetables and Brassica crops. Although several flea beetle species attack vegetable crops, the most damaging species is the pale striped flea beetle. This beetle has a very broad host range and is an important pest in all leafy vegetables, Brassica crops, carrots, beets, and cucurbits. They can also occur in field crops such as alfalfa, corn, cotton, sugar beets, and Sudan grass. Additionally, the pest can be found on several weed species, including purslane, lambsquarter, and pigweed, thus proper management of weeds in and around your plots can help with the management of the pest. On leafy vegetables and Brassica crops, pale striped flea beetle adults cause most of the damage by attacking the emerging cotyledons of direct-seeded plants and the tender new growth of transplants during stand establishment.
Organic insecticide options for the pale striped flea beetle are limited. We observed some inconsistent results from fall 2024 & 2025 trials. Results from fall 2024 trial demonstrated that some organic insecticides including Biolink (insect & bird repellent), a tank mix of Biolink + Pyganic, and a tank mix of Entrust + M-Pede provided 55, 51, and 46% suppression of pale striped flea beetle, respectively (Fig.1). However, these insecticides only provided minimal suppression of the pest in our fall 2025 trial (Fig. 2). In fall 2024, insecticide treatments were applied using chemigation through sprinklers during the last 40 minutes of germination water while, in 2025, insecticides treatments were applied at cotyledon stage using a backpack sprayer. This indicates that applying these insecticides using chemigation through sprinklers during the last 40 minutes of germination water might be the best timing to enhance the efficacy of these organic insecticides against the pale striped flea beetle. Among the insecticides evaluated in our fall 2025 trial, Captiva Prime resulted in more pale striped flea beetle suppression (Fig. 2).

Figure 1. Insecticide efficacy trial against pale striped flea beetle, fall 2024.

Figure 2. Insecticide efficacy trial against pale striped flea beetle, fall 2025.
Additional Reading Materials
1- Calvin et al. 2025. Organic-Allowed Insecticide Options for the Management of Six Major Insect Pests in Arizona’s Vegetable Crops. https://extension.arizona.edu/publication/organic-allowed-insecticide-options-management-six-major-insect-pests-arizonas
2- Palumbo, JC. 2018. Insect Management on Desert Produce and Melons: Pests at Stand Establishment. https://vegetableipmupdates.arizona.edu/sites/default/files/2021-09/180808_pests_at_stand_establishment_2018.pdf
Out in the field, water is life. Every drop counts, especially in places like the desert Southwest, where summers run hot and dry, and winters bring little or no rainfall. Many farmers are asking an important question: Does organic lettuce really use less water than conventional lettuce?
To answer that, it helps to start with the soil. Think of soil as a sponge. In conventional systems, synthetic fertilizers often deliver nutrients quickly, but over time the soil can lose some of its natural structure and ability to hold moisture. Organic systems, however, are built on compost, cover crops, and other organic matter. This added organic matter improves soil structure, making it spongier. A healthier soil sponge can soak up rainfall, hold on to moisture longer, and release it more slowly to plant roots.
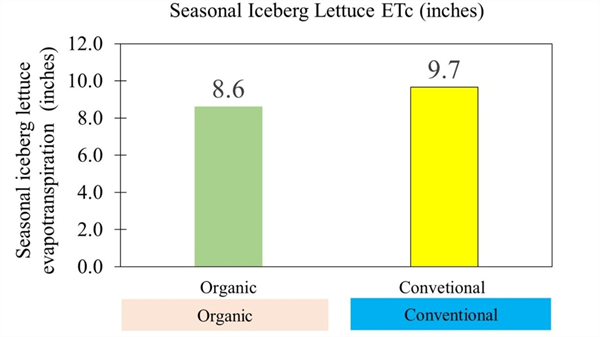
Last fall, at the Yuma Ag Center in Yuma, AZ, we conducted a field trial to measure seasonal water use in organic and conventional lettuce.
The results were encouraging. On average, organic lettuce used about one inch less water over the season compared to conventional lettuce. At first, one inch might not sound like much. But in real farm terms, it could mean skipping an entire irrigation, saving a day of labor, fuel, or electricity for pumping, and wear on equipment. That’s a meaningful saving, especially during a season when every drop counts.
Even more, these results suggest that water savings could grow when paired with improved lettuce hybrids, more efficient irrigation systems, and season-based irrigation scheduling. Each small step adds up, and together they can help farmers stretch water resources further without sacrificing yield or quality.
So yes, organic lettuce does use less water, thanks to soils that make every drop count. And in an era of tighter water supplies, that efficiency might be one of the best tools growers have
This time of year, John would often highlight Lepidopteran pests in the field and remind us of the importance of rotating insecticide modes of action. With worm pressure present in local crops, it’s a good time to revisit resistance management practices and ensure we’re protecting the effectiveness of these tools for seasons to come. For detailed guidelines, see Insecticide Resistance Management for Beet Armyworm, Cabbage Looper, and Diamondback Moth in Desert Produce Crops .
VegIPM Update Vol. 16, Num. 20
Oct. 1, 2025
Results of pheromone and sticky trap catches below!!
Corn earworm: CEW moth counts declined across all traps from last collection; average for this time of year.
Beet armyworm: BAW moth increased over the last two weeks; below average for this early produce season.
Cabbage looper: Cabbage looper counts increased in the last two collections; below average for mid-late September.
Diamondback moth: a few DBM moths were caught in the traps; consistent with previous years.
Whitefly: Adult movement decreased in most locations over the last two weeks, about average for this time of year.
Thrips: Thrips adult activity increased over the last two collections, typical for late September.
Aphids: Aphid movement absent so far; anticipate activity to pick up when winds begin blowing from N-NW.
Leafminers: Adult activity increased over the last two weeks, about average for this time of year.







