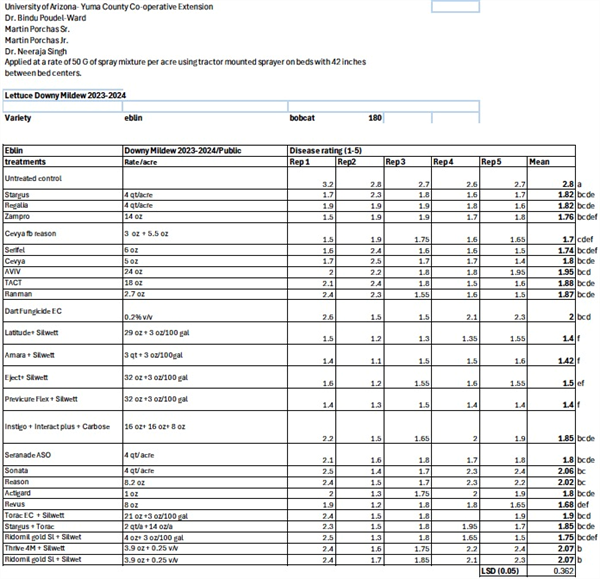
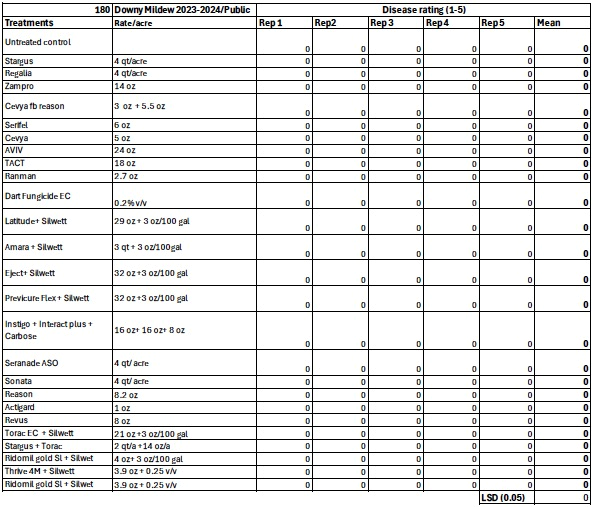
-
Aug 24, 2022Are Bagrada Bugs Still a Threat to Desert Crops?
With brassica crops now being transplanted it’s time to be on the look-out for the elusive bagrada bug. The invasive stinkbug invaded the desert southwest 12 years ago and caused significant economic damage to large acreages of Cole crops. But in the past several seasons bagrada populations have been relatively light in our experimental plots (see graph below) and only found sporadically in commercial crops. Last fall, they were almost non-existent. Why the drastic reduction? We’re not certain, but likely a combination of good insect management in nurseries and the field, and a little help from mother nature. However, we did find an abundance of adults and nymphs colonizing mustard and other brassica plots late last spring. Thus, you shouldn’t count them completely out this season; a lot will depend on how well they survived the long summer, and if there are any hitchhikers on transplants. To date, no reports of bagrada on the early transplants. But you never know, bagrada have been known to survive the summers on cotton, alfalfa, Bermuda grass, Sudan grass and weeds. So, be aware of your surroundings, especially if you have a brassica crop going in the ground next to a defoliated cotton field or recently disked Sudan field. Experience over the past years has shown us that peak adult bagrada bug abundance in the desert generally occurs on direct-seeded and transplanted brassica crops in mid-September. These peak occurrences appear to coincide with the end of the monsoon season and the much-reduced humidity. However, it’s been difficult to observe this effect lately since there has been a trend of significantly lower bagrada bug abundance since 2014, and last season was one of the lightest bagrada populations since we started tracking them in 2010.
So, what should a PCA expect for this season? We’re having an active monsoon so far, and the higher humidity could play to your advantage. We can’t say for sure when they will show up and in what numbers but keep your eyes open for adults and their feeding damage on your earliest plantings. Here are a few management tips to consider.(1) When scouting for bagrada bugs, PCAs should look mainly for fresh feeding signson new plant tissue, and adults later in the day when they are most active.
(2) Direct-seeded and transplanted crops are susceptible to bagrada bug infestations during stand establishment and up to the 6-leaf stage. After that, bagrada rarely cause damage to head forming brassicas (ie., broccoli, cabbage, cauliflower). Leafy brassicas like kale are much more susceptible to cosmetic damage throughout the season though.(3) We recommend that control be initiated immediately if 5% or more of plants have fresh-feeding signs. This can include chemigation or aerial applications with pyrethroids. Contact insecticides should be used until stands are lined out. Once pipe is pulled and adults continue to migrate into fields, consider alternating to neonicotinoid (e.g., Venom, Scorpion, Endigo) sprays.
(4) Also, growers who plant NipsIt broccoli seed (clothianidin treated) should begin to closely monitor for fresh feeding damage around 14 days after cotyledon emergence.
**** For more information on bagrada bug management on fall brassica crops go to this link: Bagrada Bug Management Tips-2022.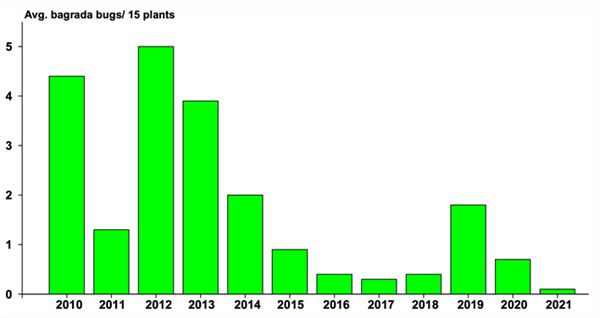
Bagrada bug abundance on untreated brassica plants during September, October, and November at the Yuma Ag Center, 2010-2021.To contact John Palumbo go to: jpalumbo@ag.Arizona.edu