
Soil is a natural component of terrestrial ecosystems, both native and agricultural. Soils are the foundation of plant and crop production systems (Brady and Weil, 2008; Parikh and James, 2012).
In terms of soil health, a good way to consider a soil system is from the standpoint of three main categories (physics, chemistry, and biology) that are fundamentally important with healthy soil function (Figure 1).
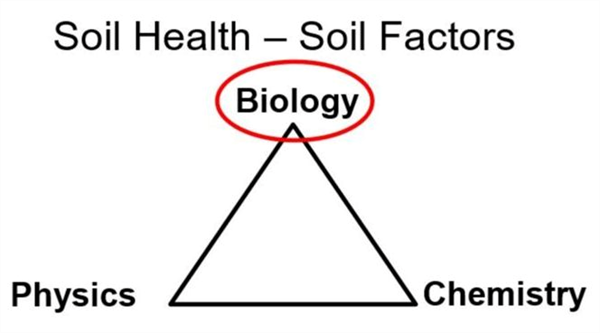
Figure 1. Soil health factors and chemical properties.
It is estimated that soil contains at least a quarter of the biological diversity (aka biodiversity) on our planet. Soils are second only to the oceans in terms of biological diversity. Billions of earthworms, nematodes, insects, fungi, bacteria, actinomycetes, viruses, and other invertebrates exist in soil naturally. These creatures produce and consume the organic material (i.e., crop residues) found in soil as part of their basic fund and collectively work to break down the organic materials that come from their bodies and plant materials into minerals and nutrients that are cycled in the soil system and support healthy conditions in the soil environment, including plant nutrients.
During the 20th century, soil scientists began to better understand the complexity of soil biology and ecology. From that work, many drugs and vaccines have been derived soil organisms. For example, antibiotics such as penicillin have been developed. Treatments for cancer such as bleomycin have come from soil and fungal infection treatments like amphotericin have also been derived from the soil microbiome. Accordingly, the biodiversity of soil still has a huge potential to provide new drugs that can help us in combating other illnesses and dealing with pathogenic and resistant microorganisms.
This tremendous biological diversity creates the foundation for the entire soil ecosystem. Soil ecosystems impact the growth of plants and animals in natural and agricultural systems. The biological composition of soils has a very strong influence on soil health.
During the 20th century soil scientists had an appreciation for the rich biological diversity in soil but they were limited in their analytical capacities (Waksman, 1936). In the past 40-50 years, soil scientists have employed an increasing level of new analytical tools that have allowed the discovery and better understanding of the number and diversity that naturally exists in soil (Sutton and Sposito, 2005).
A good description of biological diversity and its tremendous complexity can be demonstrated by consideration of the common composition of soil and the abundance of a few classes of microorganisms in one gram of soil (Figure 2).

Figure 2. Natural abundance of bacterial, actinomycetes, and fungal organisms in one
gram of soil.
It is incredible to realize that in one gram of soil there commonly exists nearly one billion individual bacterial organisms, more than a million actinomycetes organisms, and one million fungal organisms. All these organisms naturally exist in soil and these population numbers represent just one gram of soil!
Among the approximately one billion individuals in one gram of soil, there are commonly more than 10,000 different species. Soil biologists are now working to better identify individual organisms and understand their functions individually and how they function in the complexity of the soil ecosystem. We do not know all the species. Accordingly, we do not know how all these organisms are forming and functioning as a complex ecosystem. Soil ecology is a huge and rapidly expanding area of study.
We do know that soil biology and ecology are very important aspects of soil health (Figure 1). We also know that our management in the field, agronomic stewardship, has a strong influence on soil health and accordingly crop health.
Gaining a better understanding of soil health requires a better understanding of soil biodiversity and the functioning of soil ecosystems. It would be misleading to say that we have a solid understanding of the soil ecosystem function and all the managerial relationships. However, it is important to understand that working on this frontier of soil science and agriculture is critical to our efforts to maintain sustainable and productive agricultural systems for the future.
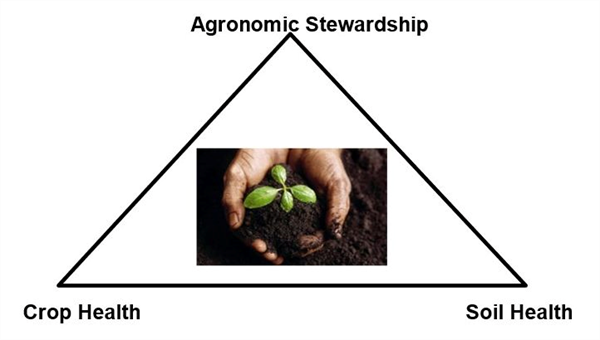
Figure 3. Interrelationship of agronomic stewardship, soil health, and crop health.
At events and in the halls of the Yuma Agricultural Center, I’ve been hearing murmurings predicting a wet winter this year…
As the Yuma Sun reported last week, “The storms of Monday, Aug. 25 [2025], were the severest conditions of monsoon season so far this year in Yuma County, bringing record-rainfall, widespread power outages and--in the fields--disruptions in planting schedules.”
While the Climate Prediction Center of the National Weather Service maintains its prediction of below average rainfall this fall and winter as a whole, the NWS is saying this week will bring several chances of scattered storms.
These unusually wet conditions at germination can favor seedling disease development. Please be on the lookout for seedling disease in all crops as we begin the fall planting season. Most often the many fungal and oomycete pathogens that cause seedling disease strike before or soon after seedlings emerge, causing what we call damping-off. These common soilborne diseases can quickly kill germinating seeds and young plants and leave stands looking patchy or empty. Early symptoms include poor germination, water-soaked or severely discolored lesions near the soil line, and sudden seedling collapse followed by desiccation.
It is important to note that oomycete and fungal pathogens typically cannot be controlled by the same fungicidal mode of action. That is why an accurate diagnosis is critical before considering treatments with fungicides. If you suspect you have seedling diseases in your field, please submit samples to the Yuma Plant Health Clinic or schedule a field visit with me.
National Weather Service Climate Prediction Center: https://www.cpc.ncep.noaa.gov/
National Weather Service forecast: https://forecast.weather.govPlans are firming up for The Desert Difference: A Showcase of AgTech Opportunities for Growing in the Desert. The two-day event will be held November 13-14th in Yuma, AZ. The first day will be a Field Day, the second will be a standard conference with keynote speakers, breakout sessions and trade booths. Details of the event and Conference Day (Day 2) activities can be found here.
The focus of this article is the Field Day which will be held Wednesday, November 13th at the Yuma Agricultural Center. Registration begins at 7:00 am and the program starts at 7:30 am (agenda below). As with our previous AgTech Field Days, the educational workshop will feature 12 of the latest automated and robotic technologies for pest control and improved vegetable production being demonstrated in the field.
This year, we’ve added a twist we think you’ll like. We’ve asked people demonstrating equipment to treat one of their plots two weeks prior to the event. This way, attendees can “see” the end result of the treatment – how well an automated weeding machine technology actually controlled weeds, for example. A second set of plots, immediately adjacent to the pre-treated plots, will be used for a live demonstration of the equipment used.
As mentioned in previous articles, we would love to showcase as many innovations as possible. All live demo slots have been filled, however there are still openings for static displays. If you are interested in being added to the program, please let me know and we will do our best to try and accommodate you. It’s an open invitation - private companies, university and government researchers are all welcome.

Fig.1. Technologies for controlling weeds “pre-treating” plots two weeks prior to the AgTech Field Day. Weed control efficacy of the pre-treated plots along with the technology being operated in the field will be shown at the event.

Fig. 2. Field Day agenda (Day 1) for The Desert Difference: A Show case of AgTech Opportunities for Growing in the Desert event. More information about the event and Conference Day activities (Day 2) can be found here.
This inquiry has been brought to us for different herbicides; How much time does this product require to safely plant lettuce?
Of course, this depends on many variables such as the management done in the crop before lettuce, texture, water applied, rate, climatic conditions presented, and many other factors.
The following table published in this Newsletter by Barry Tickes can serve as a general guideline to base Integrated Pest Management decisions and program our strategies. It’s always recommended to check the label for the product used in previous crops.

Preliminary results from a field trial that is being conducted at the Yuma Agricultural Center experimental farm to evaluate seven bioinsecticides against whiteflies in broccoli demonstrate that M-Pede (Potassium salts of fatty acids aka insecticidal soap), BotaniGard 22WP (Beauveria bassiana Strain GHA), Pyganic (Pyrethrins), Surround (Kaolin clay), and Venerate (Burkholderiaspp. Strain A396) may favor measurable control of the pest. These insecticides exhibited a reduction in whitefly adults and nymphs density relative to the nontreated control (Figure 1). The trial is ongoing, and more data will be collected to assess the efficacy of these insecticides further. Potassium salts of fatty acids are produced by adding potassium hydroxide to fatty acids found in animal fats and in plant oils. Pyrethrins is a mixture of six active compounds extracted from Chrysanthemum cinerariifolium plants. Beauveria bassiana is a naturally occurring entomopathogenic fungus. Burkholderia spp. strain A396 is a naturally occurring entomopathogenic bacteria. Kaolin clay is a soft, white, naturally occurring clay mineral.

Figure 1. Means whitefly adults (A), small whitefly nymphs (B), large whitefly nymphs (C),
and total whitefly nymphs (D) as affected by bioinsecticide applications. Bars with the
same letters are not statistically significant.
In regions like Yuma, AZ, extensive farming practices, irrigation and nitrogen (N) fertilizer management should be considered simultaneously due to the important fact that N moves in the soil with water and both variables should be managed together to enhance production efficiency. Coupled irrigation and N management strategies with the efficient irrigation method can lead to a critical approach to increase irrigation and nitrogen use efficiency (NUE) while maintaining crop yield and soil productivity and minimizing the potential for N leaching or losing. The mass of leached N during the growing season may be reduced by improved irrigation efficiency that can reduce drainage volume. For example, the surface/furrow irrigation system has greater irrigation depths and lower NUE than sprinkler irrigation systems. Moreover, the methodology of N application through split/timing applications can increase the NUE, especially when utilizing micro-irrigation and sprinkler irrigation systems. One of the primary objectives of the irrigation systems is to maximize the water storage in the root zone through uniform irrigation application and distribution and in the meantime to minimize water losses through deep percolation and surface run-off. In addition, irrigation systems have been utilized to apply fertilizers (fertigation) throughout the season. Generally, these systems provide a way to supply adequate N (allows small dosage application) to the crop in-season and those systems can deliver the desired nutrition amount to the crop at any crop stage with a high efficiency and distribution uniformity. In other words, a given irrigation system has the potential to reduce the fertilizer inputs and the production costs, reduce foliar disease, and minimize leaf wetness as well as reduce the weeds.
The three most commonly used irrigation methods/systems are (i) surface (gravity), (ii) sprinkler (including center pivot), and (iii) micro-irrigation. For each of the methods, there a different management processes and the uniformity of water applications as well as infiltration dynamics, which influence the efficiency of the system as well as the efficiency of N applications. Worldwide, low NUE is one of the most important challenges for researchers, farmers, and agencies, and it is on average quite low in both organic and conventional agricultural systems, including in developed nations. It is reported that globally, pre-plant N is most commonly applied, which may lead to poor synchrony between N and crop demand, contributing to low NUE. Applying N at a uniform rate is another factor of low NUE, because the available N level for crop uptake may vary between the fields and within a given field due to the spatial variability in soil characteristics and temporal characteristics due to environmental factors.
Timing nitrogen applications for lettuce is key to maximizing nutrient management efficiency, though it can be challenging if growers are constrained by time. To minimize nutrient loss, it's best to avoid pre-plant nitrogen applications, especially in the fall. At planting, apply a starter fertilizer, positioning it below and to the side of the seed row. The first sidedress application should occur after thinning at the 2-4 leaf stage, but only if soil nitrate-nitrogen is below the critical level. A second application is recommended a few weeks later at the cupping stage, contingent on soil nitrate levels. These applications should be carefully timed and adapted based on soil conditions to ensure effectiveness. Aligning nitrogen applications with the crop’s demand not only enhances nitrogen use efficiency but also helps in managing tight schedules, reduces environmental impacts, and optimizes lettuce yield and quality (Figure 1).

Figure 1: Harvesting in the Organic/Conventional Lettuce Production Field at the Valley
Research Center, University of Arizona Yuma Agricultural Center, Yuma, Arizona.
Results of pheromone and sticky trap catches can be viewed here.
Corn earworm: CEW moth counts down in most over the last month, but increased activity in Wellton and Tacna in the past week; above average for this time of season.
Beet armyworm: Moth trap counts increased in most areas, above average for this time of the year.
Cabbage looper: Moths remain in all traps in the past 2 weeks, and average for this time of the season.
Diamondback moth: Adults decreased to all locations but still remain active in Wellton and the N. Yuma Valley. Overall, below average for January.
Whitefly: Adult movement remains low in all areas, consistent with previous years.
Thrips: Thrips adults movement decreased in past 2 weeks, overall activity below average for January.
Aphids: Winged aphids are still actively moving, but lower in most areas. About average for January.
Leafminers: Adult activity down in most locations, below average for this time of season.