
Nutrient Mobility Concept
In a recent article published in this newsletter on 27 November 2024, Volume 15, No. 24, I presented an article addressing soil health and the Bray Nutrient Mobility Concept in relation to mobile nutrients (Silvertooth, 2024). In this article, the concept of nutrient behavior in soil-plant systems focuses on the immobile plant nutrients.
Plant nutrient management is strongly dependent on nutrient mobility in the soil. Nutrient mobility in the soil is different among the essential plant nutrients and nutrient management in the field needs to take this into account.
In 1954, Dr. Roger H. Bray at the University of Illinois proposed a nutrient mobility concept that has proven to be very important in the management of nutrients for optimum efficiency (agronomically, economically, and environmentally). Bray essentially simplified all soil nutrient chemistry to the fact that some plant nutrients are mobile in the soil and some are not. (Bray, 1954; Raun, 2017; Warren et al., 2017, Havlin et al. 2014; Troeh and Thompson, 2005).
Mobile Nutrients and the Root System Sorption Zone
Mobile plant nutrients in the soil move with the soil water. Thus, plants can extract mobile nutrients from a large volume of soil beyond the direct root system. Accordingly, plants take up mobile nutrients from a “root system sorption zone” (Figure 1). This gives plants the capacity to utilize most of the mobile nutrients in the root system sorption zone as those nutrients will move to the plant roots with the soil water as it is taken up by the plant (Silvertooth, 2024).
We consider the mobile plant nutrients to be nitrogen (N), sulfur (S), boron (B), and chlorine (Cl). These mobile plant nutrients are taken up by the plant in the following forms: nitrate-nitrogen (NO3--N), sulfate-sulfur (SO42- - S), boric acid (H3BO3) and borate ions (BO33- - B), and chlorine is taken up as the chloride ion (Cl-).

Figure 1. The root system sorption zone and an illustration of the large volume of soil
from which plants extract mobile nutrients.
In a crop field where many plants are growing together, there are commonly root system sorption zones commonly overlap. Therefore, the root system sorption zones for adjoining plants are competing for water and mobile nutrients, (Figure 2). This is one of the main reasons that appropriate plant populations are important for optimum yield.
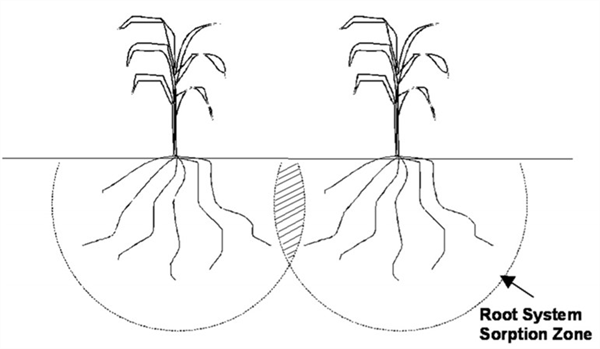
Figure 2. Competition among plants brought about by increasing yield goal.
Immobile Nutrients
Plant nutrients that are immobile in the soil include phosphorus(P), potassium (K), calcium (Ca), magnesium (Mg), iron (Fe), zinc (Zn), manganese (Mn), copper (Cu), and molybdenum (Mo). Immobile nutrients do not move as freely in the soil solution as the mobile nutrients do. These nutrients interact more directly with soil colloids and root surfaces.
Immobile nutrients are absorbed by the plant from the soil and soil solution that is directly next to the root surface. Plant roots must grow through the soil volume to come into direct contact with the immobile nutrients.
Thus, only a small volume of soil and soil solution that immediately adjacent to the root surface will be involved in providing immobile nutrients to the plant. Figure 3 describes this soil volume and plant root interface as the root surface sorption zone.

Figure 3. The root surface sorption zone and an illustration of the small volume of soil
from which plants extract immobile nutrients.
In the case of immobile nutrients, the entire soil volume is not as important as the soil colloid surfaces and soil solution next to the root surface. The concentration of immobile nutrients on the soil colloids immediately next to the root surface is a critical of the root system sorption zone.
Since only a thin layer of soil surrounding and in direct contact with the plant roots are involved in supplying immobile nutrients to the plant, there is little or no competition among plants for immobile nutrients. Competition among plants only occurs at points where roots from adjacent plants come in direct contact with one another (Figure 4).
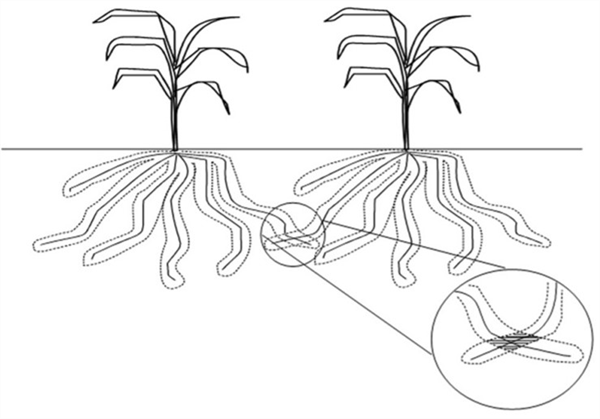
Figure 4. Limited competition among plants for immobile nutrients.
Due to this manner of immobile nutrient behavior and interaction with plant roots, the supply or concentration of immobile nutrients such as phosphorus (P) or potassium (K), is not dependent on a yield goal. In the case of immobile nutrients, the overall soil concentration of the immobile nutrients is most important, and these nutrients are not moving readily with soil water. If the immobile nutrient supply in the soil is adequate for optimum yield of a crop, the healthy plant root system can explore new soil volume and extract the nutrient sufficiently, such as phosphorus (P) or potassium (K).
The nutrient mobility concept and these basic illustrations can help us understand the basis for some common observations and resultant crop management practices. Fertilizers with immobile plant nutrients are more effective when they are incorporated into soil and particularly in soil zones where there is a high probability of plant roots encountering the immobile nutrients.
Banded applications of immobile nutrients are generally more effective than the same rates broadcast and incorporated into the soil. In contrast, mobile nutrients like nitrogen (N) can be broadcast and moved into the root system sorption zone by water.
Soil tests for immobile nutrients do not normally change much from year to year and this is true irrespective of the crop yields from the previous season or fertilizer rate. This is because most of soil volume was not in direct contact with the plant roots. Soil concentrations of immobile nutrients do not usually change rapidly but they can be slowly mined out of the soil by a series of crops without proper fertilization.
Continued or over-applications of immobile nutrient fertilizers, such as phosphorus (P), will cause a buildup of that nutrient in the soil. This is because only a small fraction (commonly 15-20% for most crops) of the nutrient or fertilizer comes into direct contact with the plant roots. The remaining amount of fertilizer interacts with the soil.
Appropriate soil tests that are properly correlated and calibrated with crop-specific response categories are important in evaluating immobile plant nutrient status. Immobile nutrient levels in the soil are commonly expressed in terms of percent sufficiency to produce a specific crop based on appropriate soil test results.
Our goal in plant nutrition management is to achieve the highest levels of efficiency (agronomically, economically, and environmentally) in the field as possible
References:
Bray, R.H.1954. A Nutrient Mobility Concept of soil-plant relationships. Soil Sci. 78(1), p. 9-22.
Havlin, J.L., Beaton, J.D., Tisdale, S.L. and Nelson, W.L. 2014. Soil Fertility and Fertilizers; An Introduction to Nutrient Management. 6th Edition, Prentice Hall, Upper Saddle River, NJ.
Silvertooth, J.C. 2024. Soil Health - Bray’s Nutrient Mobility Concept and Mobile Plant Nutrients University of Arizona Vegetable IPM Newsletter, Volume 15, No. 24,
Raun, W.R. 2017. In: Warren et al. 2017. Oklahoma Soil Fertility Handbook, Id:E-1039
Troeh, F.R. and Thompson, L.M. (2005) Soils and Soil Fertility. Sixth Edition, Blackwell, Ames, Iowa, 489.
Warren, J., H. Zhang, B. Arnall, J. Bushong, B. Raun, C. Penn, and J. Abit. 2017. Oklahoma Soil Fertility Handbook. Id: E-1039
Weil, R.R. and Brady, N.C. (2017) The Nature and Properties of Soils. 15th Edition, Pearson, New York.
Plans are firming up for The Desert Difference: A Showcase of AgTech Opportunities for Growing in the Desert. The two-day event will be held November 13-14th in Yuma, AZ. The first day will be a Field Day, the second will be a standard conference with keynote speakers, breakout sessions and trade booths. Details of the event and Conference Day (Day 2) activities can be found here.
The focus of this article is the Field Day which will be held Wednesday, November 13th at the Yuma Agricultural Center. Registration begins at 7:00 am and the program starts at 7:30 am (agenda below). As with our previous AgTech Field Days, the educational workshop will feature 12 of the latest automated and robotic technologies for pest control and improved vegetable production being demonstrated in the field.
This year, we’ve added a twist we think you’ll like. We’ve asked people demonstrating equipment to treat one of their plots two weeks prior to the event. This way, attendees can “see” the end result of the treatment – how well an automated weeding machine technology actually controlled weeds, for example. A second set of plots, immediately adjacent to the pre-treated plots, will be used for a live demonstration of the equipment used.
As mentioned in previous articles, we would love to showcase as many innovations as possible. All live demo slots have been filled, however there are still openings for static displays. If you are interested in being added to the program, please let me know and we will do our best to try and accommodate you. It’s an open invitation - private companies, university and government researchers are all welcome.

Fig.1. Technologies for controlling weeds “pre-treating” plots two weeks prior to the AgTech Field Day. Weed control efficacy of the pre-treated plots along with the technology being operated in the field will be shown at the event.

Fig. 2. Field Day agenda (Day 1) for The Desert Difference: A Show case of AgTech Opportunities for Growing in the Desert event. More information about the event and Conference Day activities (Day 2) can be found here.
In a letter from the EPA to AMVAC dated May 2, 2024 that you can find by clicking here: https://www.regulations.gov/document/EPA-HQ-OPP-2011-0374-0116 EPA "thanks AMVAC for voluntarily proposing to discontinue DCPA use on onions".
If DCPA is not available next season our options for onion weed control are reduced. Please read the work from Carl Bell https://escholarship.org/content/qt91w4d06x/qt91w4d06x.pdf?t=lnryxp&v=lg regarding combining bensulide and pendimethalin for weeds control in onions. He did this research due to a similar situation with Dacthal in 1996.
We tested last season 2 qts of Prefar and 0.5 pt of Prowl and the weed control looked promising. There appears to be a synergystic effect between these products. Some phytotoxicity was observed and stand was affected. Bell reported (2001) that "a reduced crop stand does not always equal a reduced yield, since onions compensate to some extent by producing larger bulbs".
Some PCAs are planning IPM strategies and are suggesting for green onions: "Apply Goal early post emergence with clethodim if you have grasses then Prowl at layby".
What do you think? keep sending your comments to the Veg IPM Team and let us know what you think.

A species' persistence requires the ability to adapt to the biotic and/or abiotic conditions present within its environment. This adaptation may occur through natural selection, which is the process by which organisms that are better adapted to their environment tend to survive and have better fitness. Natural selection is believed to be the motor of evolution. Evolution has occurred within all groups of organisms, including plant and animal populations.
Plants have evolved resistance in many circumstances, including resistance to pests and pathogens attacks. In response to insect herbivory attacks, plants have been using chemical defenses to resist. Consequently, to maximize their fitness, plant-feeding insects have co-evolved with plants to overcome plant defenses utilizing an array of strategies. Insects have evolved the ability to detoxify plant chemicals used for defenses and use the compounds as cues that favor the detection of the plant host. Insects have also evolved an adjusted sensory system allowing host cues detection and a nervous system that is able to integrate inputs from sensory neurons. The enhancement in the sensory and nervous systems allows the detection and avoidance of toxic plants as well as the excretion, sequestration, and degradation of plant toxins. Additionally, herbivory insects utilize target-site mutation, cuticular, humoral, and cellular defenses against plant chemical defenses. Moreover, insects have evolved to resist predation, parasitism, and pathogen attacks by means of a series of mechanisms, including cuticular adjustment, adaptive behavior, and chemical defenses.
With the intensive use of pesticides to manage agricultural pests, insect pests have evolved resistance to an array of insecticides using a variety of mechanisms. This type of evolution has been described as field-evolved resistance, which is a “genetically based decrease in susceptibility of a population to a toxin caused by exposure to the toxin in the field”. This is due to strong selection pressure that favors rapid evolution of resistance. For example, the widespread adoption of Bt crops in the U.S. has led to field-evolved resistance of corn earworm, also known as cotton bollworm, against Bt toxins. In some regions, Texas, for example, cotton bollworms being exposed to the Bt toxins in both corn and cotton throughout the year have been subjected to a high selection pressure, causing the pest to become quickly resistant to Bt toxins.
The use of beneficial arthropods to manage insects can favor a decrease in insecticide use and consequently reduce the selection pressure caused by pesticides. Although the evolution of resistance to predators and parasitoids tends to be prohibited by some factors (special and temporal refuges from enemies’ attacks, reciprocal evolution by control agents, and contrasting selection pressure from enemy species), the evolution of resistance to biological control agents has been reported for several insect pests including Argentine stem weevil, greater wax moth, and fruit fly. This is likely due to reduced plant and natural enemy diversity caused by intensive large-scale agriculture.
Several factors may play a role in the development of resistance. Large-scale homogenous agricultural systems do not allow enough refuges to sustain the susceptible strains, which would then mate with the resistant strains to dilute the resistance genes and maintain the susceptibility of the pest populations. Additionally, low biodiversity within the natural enemy population may favor the selection pressure. Coevolutionary arms races may play a significant role in that this may favor one participant in mutation and recombination rates.
Mechanisms of resistance:
Physiological resistance: Insects use physiological processes to become resistant to enemies and insecticides. In a pesticide use context, physiological resistance is defined as the capacity of an insect population to survive after being exposed to a concentration of insecticide that is known to be able to kill the totality of the population completely. However, the physiological process can also favor resistance against non-pesticide control methods. For instance, the fruit fly (Drosophila melanogaster) uses encapsulation to protect itself from koinobiont endoparasitoids. The encapsulation is a cellular immune response that follows three major stages, including the recognition of the parasitoid eggs as foreign, increasing the amount of circulating hemocytes that are produced by the lymph glands, and the lysis of the crystal cells allowing the release of prophenoloxidase which results in the melanization of the capsule surface.
Another way insects become resistant is through mutation in the target site of the toxicant. This physiological process can lead to resistance in insects against both plant defenses (toxic compounds released by the plant to protect itself from herbivory) and insecticides. This mutation can lead to target site insensitivity, meaning that even though the insect is being exposed to the toxic molecule, there will be no or reduced binding of the molecule to the target site, making the molecule ineffective. This mechanism of resistance is very common in many insecticide-resistant insect pests. Insects can also become resistant to toxic compounds from plants and to insecticides by evolving the ability to undergo detoxification of certain toxicants after exposure. This ability is also conferred by a series of mutations allowing the resistant insect to increase their enzyme production, which consequently increases their enzymatic activity and causes a rapid degradation of the toxicant into a nontoxic compound. This mechanism is also known as metabolic resistance.
Behavioral resistance: Many insect species have become resistant to certain host plants that use defense compounds to prevent herbivory through their plant selection and feeding behavior. For this behavior to occur, they must evolve the ability to detect toxic plants, which can be determined genetically or through a series of learning processes. Some other insects evolved the ability to deactivate or suppress the toxin produced by the plant hosts. For instance, the cotton bollworm uses its saliva, which is a gluco-oxidase, to cause a reduction in the level of nicotine produced in tobacco leaves. Other insects, when they feed on toxic plants, can excrete a significantly large amount of the accumulated toxic compound. Some insects even sequester the toxic compounds and use them for their own defense against predators and pathogens. Some insects that are hosts for parasitoids use a very effective behavioral resistant strategy by avoiding parasite contact or detection by choosing to niche away from the parasitoids or by choosing to locate themselves near a deterrent. Using this behavior, these insects are not directly resistant to the attackers but use what is present in their environment as tools to resist parasitism. Some other insects use alternative strategies, such as cryptic coloration or masquerade, to prevent their detection by predators and/or parasitoids. In this situation, they disguise themselves as something dangerous or unwanted to avoid being prayed on or parasitized.
Cuticular resistance: Insects depend heavily upon cuticular defenses to resist pathogens, parasitism, predation, and insecticides. For instance, to resist insecticide penetration, they develop a barrier in the outer layer of the cuticle either by changing the composition of the cuticle or by thickening it. This causes the toxicant to be penetrated slowly, consequently slowing the absorption of the contaminants to the insect bodies, where actions will take place.
Although the development of resistance is mostly beneficial for insects, there are some fitness costs associated with that. Physiological resistance, behavioral resistance, and cuticular resistance require the use of a large amount of energy; some energy that would have been allocated for growth, development, and reproduction is likely to be reduced, which would consequently reduce the fitness of the insect. Thus, fitness cost may cause an evolutionary constraint, which may reduce the rate or even prevent the evolution of resistance from occurring. Given that, an increase in resource availability is likely to favor the rate at which evolution occurs within a population.
In conclusion, resistance in insects can occur in a diversity of forms, and several factors may cause resistance to occur within insect populations. Additionally, while insect populations are more likely to be resistant to insecticide in large-scale agricultural systems, they can also become resistant to biological control agents, which underscores the importance of integrated pest management programs. The rate at which resistance occurs in a population closely depends on the intensity of selection pressure to which the insect populations are exposed. Thus, the more intense the selection pressure the quicker the populations will evolve resistant.
References:
1- Ali, J. G, and A. A. Agrawal. 2012. Specialist versus generalist insect herbivores and plant defense. Trends in Plant Science. 17: 293-302.
2- Balabanidou, V., L. Grigoraki, and J. Vontas. 2018. Insect cuticle: a critical determinant of insecticide resistance. Current Opinion in Insect Science 2018, 27:68–74.
3- Berenbaum, M.R. 1986. Target site insensitivity in insect-plant interactions. In: Brattsten, L.B., and S. Ahmad. (eds) Molecular aspects of insect-plant associations. Springer, Boston, MA. https://doi.org/10.1007/978-1-4613-1865-1_7
4- Boots, M. 2010. The Evolution of Resistance to a Parasite Is Determined by Resources. The American Naturalist. 178: 214-220.
5- Castagnola, A., and J. L. Jurat-Fuentes. 2016. Intestinal regeneration as an insect resistance mechanism to entomopathogenic bacteria. Current Opinion in Insect Science. 15:104–110.
6- Chareonviriyaphap, T., M. J. Bangs, W. Suwonkerd, M. Kongmee, V. Corbel, and R. Ngoen-Klan. 2013. Review of insecticide resistance and behavioral avoidance of vectors of human diseases in Thailand. Parasites & Vectors. 6: 280.
7- Dang, K., S. L. Doggett, G. V. Singham, and C-Y. Lee. 2017. Insecticide resistance and resistance mechanisms in bed bugs, Cimex spp. (Hemiptera: Cimicidae). Parasites & Vectors. 10:318, DOI 10.1186/s13071-017-2232-3
8- Després, L., D. Jean-Philippe, and C. Gallet. 2007. The evolutionary ecology of insect resistance to plant chemicals. TRENDS in Ecology and Evolution. 22: 298-307.
9- Dubovskiy, I. M., M. M. A. Whitten, O. N. Yaroslavtseva, C. Greig, V.Y. Kryukov, E. V. Grizanova, K. Mukherjee, A. Vilcinskas, V. V. Glupov, and T. M. Butt. 2013. Can insects develop resistance to insect pathogenic fungi? PLoS ONE. 8: e60248. doi:10.1371/journal.pone.0060248
10- Fellowes, M. D. E., and H. C. J. Godfray. 1999. The evolutionary ecology of resistance to parasitoids by Drosophila. Heredity. 84:1-8.
11- Ferré, J., and J. V. Rie. 2002. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annual Review of Entomology. 47:501–33.
12- Heidel-Fischer, H., and H. Vogel. 2015. Molecular mechanisms of insect adaptation to plant secondary compounds. Current Opinion in Insect Science. 8: 8–14.
13- Mills, N. J. 2017. Rapid evolution of resistance to parasitism in biological control. PNAS. 114: 3792-3794.
14- Ryan, M. F., and O. Byrne. 1988. Plant-insect coevolution and inhibition of Acetylcholinesterase. Journal of Chemical Ecology. 14: 1965-1975.
15- Tabashnik, B. E. and Y. Carrière. 2010. Field-evolved resistance to Bt cotton: Helicoverpa zea in the US and pink bollworm in India. Southwest. Entomol. 35: 417–424.
16- Tomassetto, F., J. M. Tylianakisb, M. Realed, S. Wrattene, and S. L. Goldsonet. 2017. Intensified agriculture favors evolved resistance to biological control. PNAS.114: 3885–3890.