
The cycling of nitrogen (N) in the soil is one of the most important aspects of soil health. Nitrogen cycling in the soil includes a multitude of interactions with microbes and plants.
The basics of soil N were described in a recent article posted in this UA Vegetable IPM newsletter (Silvertooth, 2024). The overall N reactions in the soil system are described in the N Cycle, Figure 1.
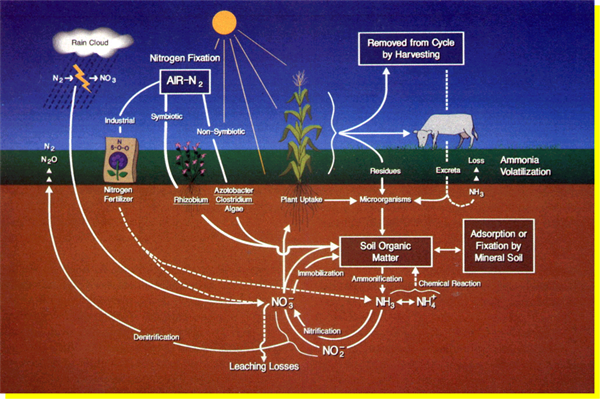
Figure 1. The nitrogen cycle.
Most of the N in soil is present as organic N in the soil organic matter. As a general estimate, there are approximately 1,000 pounds N per acre of this form for every one percent organic matter in the soil. However, soil organic matter (SOM) is relatively stable and resistant to further decay. So, most of the organic N in the soil is not biologically available during any given growing season (Havlin, et al. 2014;Thompson and Troeh, 2005; Warren, et al., 2017; and Weil and Brady, 2017).
Normally, about two percent of the N from SOM will be released each year to mineral forms when soil is cultivated. Arizona soils typically have approximately 1%organic matter. Thus, 10 to 20 pounds of N per acre is a typical amount of N present in unfertilized soils after cultivation and seed bed preparation.
We commonly assume that 1 acre-furrow slice of soil (soil that is 6 inches deep covering an area of 1 acre (43,560 ft.2))contains approximately 2 million pounds of soil. Thus, a soil in Arizona with 1% organic matter will have approximately 20,000 lbs. of SOM per acre and that will contain about1,000 lbs. of total N (5% N in the SOM), most of that being in an organic form that is not directly plant-available.
If 2% of the N in the SOM does mineralize in one year, that would release approximately 20 lbs. of N per acre per year. Accordingly, it is common to find 10 to 20 pounds of mineral plant-available N (NO3--N) per acre. That is a typical amount of mineral plant-available N present in unfertilized soils after cultivation and seed bed preparation. Higher levels of residual NO3--N is commonly a remnant of prior fertilizations.
Nitrogen Mineralization and Immobilization
Because N release from SOM is dependent upon decay by microorganisms, which themselves require N, the amount of N available for a crop is in constant flux. Unlike plants, which get their carbon (C) as carbon dioxide (CO2) from the air, many microorganisms get C from decaying organic matter.
Nitrogen availability depends upon the relative amount of C and N in the SOM, its resistance to decay, and the environmental conditions supporting microbial activity. Figure 2 illustrates how mineral N becomes more concentrated in SOM as soil organic matter decays.

Figure 2. The carbon to nitrogen ratio (C:N) declines and narrows as residues decay
until mineral nitrogen finally becomes available.
(Source: B. Raun In: Warren, et al., 2017)
Note that mineral N is not released during the first stages of decay. This is because N that is released is immediately consumed by active microorganisms. With time, remaining SOM becomes more resistant to decay, microorganisms die off, and there is more mineral N present than can be consumed by the few active microorganisms.
The first step of measurable mineral N release from SOM is usually in the form of ammonia NH3. The ammonia readily reacts with soil moisture to form ammonium NH4+. These two reactions can be stated simply as:
1) Organic nitrogen → NH3 (gas)
2) NH3 + H2O → NH4+ + OH- (ammonia + water → ammonium + hydroxide)
The process of converting or transforming N from organic compounds to inorganic compounds is called mineralization. This results in increasing N available for crops. The N form that is directly available to plants is nitrate-N (NO3--N).
When the reverse happens, plant-available N, primarily nitrate-N (NO3--N) is absorbed by crops or microorganisms. This process is called immobilization and results in a decrease in the amount of N in the soil solution that is immediately available for crops.
These processes of mineralization and immobilization and their interacting nature for a typical field soil situation are illustrated in Figure 3 starting with 2,060 lbs. of total soil N in this case partitioned among the SOM, crop residues, and mineral N pools.
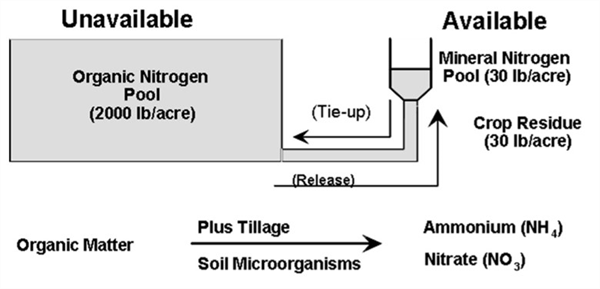
Figure 3. Interacting pools of soil nitrogen.
(Source: B. Raun In: Warren et. al., 2017)
Approximately 98 percent of the soil N is unavailable for plant uptake. This large reservoir of organic N provides an important buffer against rapid changes in available N and plant stress. The small reservoir of mineral N can often be slowly replenished by mineralization (Figure 2.2).
Supplemental N as fertilizer usually must be added to support high, economic crop production levels when native levels of soil N mineralization are not sufficient. This is common in Arizona agricultural soils.
Example Case:
Immediately following fertilization with 120 pounds N, the system may be illustrated by Figure 4a. Note the increase of 120 pounds of N in the mineral N pool in addition to the original 60 lbs. of mineral N in this case (Figure 3), for a total of180 lbs. of mineral N. While the organic N pool is static at 2000 lb./acre in this example. In this case there is a total of 2,180 lbs. Nin the soil.
Addition of fertilizer N will stimulate microorganism activity, resulting in consumption of mineral N by soil microbes as they attack and break down some of the crop residues (immobilization) as illustrated in Figure 4b. Note that the total amount of N among the three pools (mineral, organic, and crop residues) is the same at 2,180lbs. N/acre.
The immobilized N will be present as microbial tissue and other new material in the organic pool. Note that the N present in the crop residue has dropped from 30 to 20 lbs. N/acre in this example due to microbial decomposition.
An important point to follow in this example is the transformations taking place with the consistent base of 2,180 lbs. of N partitioned into various forms. This is a rather simplified example, but it illustrates the natural dynamics taking place in the soil-plant system with the cycling of N and the challenges of managing N fertilizers effectively.
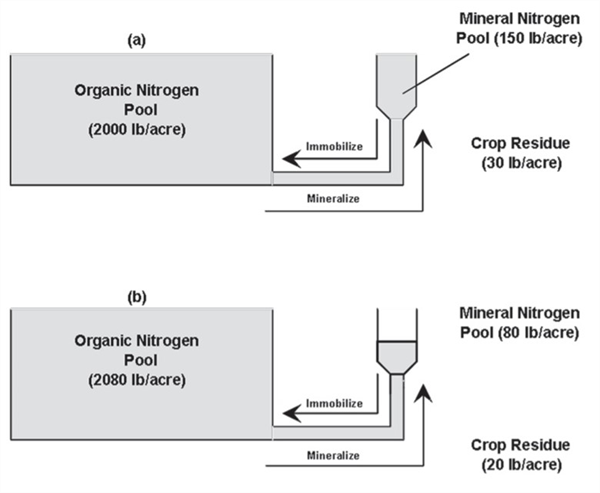
Figure 4. Relative amounts of organic and mineral nitrogen in soil immediately after
fertilizing (a) and several days after active immobilization (b).
As indicated by the two arrows pointing in opposite pathways, mineralization and immobilization are both taking place simultaneously. Immobilized fertilizer N will again become available in a few weeks if conditions favor crop uptake.
This exchange in N forms is often referred to as the mineralization-immobilization transformation (MIT) process and it is a core element of N cycling and management for crop production.
There is a constant flux taking place in soils among these pools of soil N through the MIT process and it is important to be aware of and understand this process when managing fertilizer N inputs for crop production.
References
Havlin, J.L., Beaton, J.D., Tisdale, S.L. and Nelson, W.L. 2014. Soil Fertility and Fertilizers; An Introduction to Nutrient Management. 6thEdition, Prentice Hall, Upper Saddle River, NJ.
Silvertooth, J.C. 2024. Soil Health – Nitrogen. University of Arizona Vegetable IPM Newsletter, Volume 15, No. 22,
Troeh, F.R. and Thompson, L.M. (2005) Soils and Soil Fertility. Sixth Edition, Blackwell, Ames, Iowa, 489.
Warren, J., H. Zhang, B. Arnall, J. Bushong, B. Raun, C. Penn, and J. Abit. 2017. Oklahoma Soil Fertility Handbook. Published Apr. 2017; Id: E-1039
Weil, R.R. and Brady, N.C. (2017) The Nature and Properties of Soils. 15thEdition, Pearson, New York.
Last week, we initiated our first on-farm demonstration of soil steaming of the season with our self-propelled steam applicator. The machine is designed to inject steam into the soil and raise soil temperatures to levels sufficient to kill weed seed and soilborne pathogens (140°F for > 20 minutes). After the soil cools (< ½ day), the crop is planted into the disinfested soil.
In this trial, we are examining the viability of soil steaming for controlling weeds in organic carrot at the field scale level (plot size > ½ ac). The machine performed well in that it was able to reach target soil temperatures at reasonable travel speeds (> 0.4 mph), provide uniform temperature distribution across the bed and form nicely shaped beds suitable for subsequent planting. Stay tuned for reports of weed control efficacy, crop yield and overall profitability as compared to the grower standard.
We are seeking collaborators to conduct similar field-scale trials/demos in Yuma, AZ. The primary objectives are to assess the viability of soil steaming at the field-scale level and obtain grower feedback on the device’s commercial potential. The machine can be adjusted to work with most bed configurations including narrow (40”, 42”) and wide (80”, 84”) beds, and is suitable for use in conventional or organic crops (soil steaming is organically compliant). To date, the device has been successfully trialed in iceberg lettuce, romaine lettuce, baby leaf spinach and carrot crops.
If you are interested in an on-farm demo of soil steaming, please let me know. I’d be happy to work with you.
Fig. 1. On-farm demonstration of a self-propelled steam applicator for weed and
disease control
Acknowledgements
This project is sponsored and funded in part by the Arizona Specialty Crop Block Grant Program and the Propane Education and Research Council (PERC). We greatly appreciate their support.
Boerhavia coulteri is a flowering plant that belongs to the Nyctaginaceae or Four O'clock family known by the common name Coulter's spiderling. Its native to the desert areas of the SW in the US and North of Mexico. It is a summer annual or perennial weed producing an erect or creeping stem up to 0.7-0.8 m in length. Grows in disturbed areas, ditch banks, and roadsides.

The cotyledons are oblong,1.0-2.5 cm.
Plants are slightly pubescent with sticky resin glands toward the bases. The leaves are lance-shaped, somewhat triangular, pointed, sometimes wavy or rippled along the edges, and 5 centimeters in maximum length1.
We have this weed in our Yuma County AZ and very abundantly in the sandy soils of the Yuma Mesa.
I added a table from a weed control experiment conduced for Boheravia erecta.
The Research Paper called :” The effect of herbicide tank mix on the weed species diversity
in sugarcane (Saccharum officinarum)” mentions: “All the herbicide mixes significantly controlled Boheravia with Pendimethalin. Being Pendimethalin plus Atrazine the best treatment as shown in the table below.
Aphids are sap-sucking insects that depend on the nutritional content of the sap ingested from the plant hosts for proper growth and development. Nitrogen availability is one of the most important factors in the development of herbivore populations. Excessive application of nitrogen fertilizer to crops is likely to increase insect pests feeding preference and consumption resulting in the survival, growth, and reproduction of the pests. This particularly affects aphids where excessive nitrogen application to host crops such as lettuce, wheat, sorghum, etc. may boost their populations by enhancing their growth and development, thus reducing their generation time, resulting in an increase in the number of generations and density during the cropping season.
Report from a study conducted on Arugula shown that excessive supply of nitrogen increased green peach aphid density. In some situations, high nitrogen levels in plant tissue can decrease resistance and increase susceptibility to aphids’ attacks. Given that, adequate management of fertilizer like nitrogen can tremendously help to manage aphids which are difficult to control pests specifically in organic lettuce production. In addition to pest management, effective fertilizer usage can also result in economic and environmental benefits.
Like fertilizer management, water management is also very important for effective pest control. Water availability around plant roots affects the rate at which nutrients are
absorbed by the plants. Thus, an increase in water availability will increase nitrogen uptake which can affect the population dynamic of aphids. Additionally, with high water availability there is an increase in phloem pressure making food more accessible to sap-sucking insect pests. Supplying the required amount of water using appropriate irrigation methods and irrigation scheduling can be beneficial for pest management. Although this practice is not likely to completely prevent infestation of aphids, it can surely play a role in reducing the density of aphid populations on crops.

Figure 1. Aphid selection of host plants: (a) The migrating aphid’s choice of landing on a particular plant depends on receiving the plant-reflected wavelengths (between about 500 nm and 600 nm); upon landing, antennal receptors detect the plant surface volatiles for initial assessment. (b) After making contact with the plant surface, the aphid briefly and tentatively pierces the epidermis using its stylet (<1 min) and ingests a small quantity of plant sap for further evaluation by a gustatory organ in the epipharyngeal area. (c) If the initial assessment is favorable, the aphid penetrates the epidermis to pierce the mesophyll and parenchyma tissues and briefly ingests more sap from vacuoles for additional evaluation and to determine the appropriateness of further ingestion (<1 min). (d) Upon identifying the host plant, the
aphid pierces the epidermis of the leaf and passes through the intercellular air spaces of the mesophyll cells using its stylet to reach the sieve tube element in plant phloem, releasing salivary enzymes to protect the mouthparts and prevent plant tissue repair, enabling continuous sap consumption. If ingestion in the sieve tube exceeds 10 min, the host plant is deemed suitable (Xia et al. 2023).
Selected References: