
Nutrient Mobility Concept
In a recent article published in this newsletter on 27 November 2024, Volume 15, No. 24, I presented an article addressing soil health and the Bray Nutrient Mobility Concept in relation to mobile nutrients (Silvertooth, 2024). In this article, the concept of nutrient behavior in soil-plant systems focuses on the immobile plant nutrients.
Plant nutrient management is strongly dependent on nutrient mobility in the soil. Nutrient mobility in the soil is different among the essential plant nutrients and nutrient management in the field needs to take this into account.
In 1954, Dr. Roger H. Bray at the University of Illinois proposed a nutrient mobility concept that has proven to be very important in the management of nutrients for optimum efficiency (agronomically, economically, and environmentally). Bray essentially simplified all soil nutrient chemistry to the fact that some plant nutrients are mobile in the soil and some are not. (Bray, 1954; Raun, 2017; Warren et al., 2017, Havlin et al. 2014; Troeh and Thompson, 2005).
Mobile Nutrients and the Root System Sorption Zone
Mobile plant nutrients in the soil move with the soil water. Thus, plants can extract mobile nutrients from a large volume of soil beyond the direct root system. Accordingly, plants take up mobile nutrients from a “root system sorption zone” (Figure 1). This gives plants the capacity to utilize most of the mobile nutrients in the root system sorption zone as those nutrients will move to the plant roots with the soil water as it is taken up by the plant (Silvertooth, 2024).
We consider the mobile plant nutrients to be nitrogen (N), sulfur (S), boron (B), and chlorine (Cl). These mobile plant nutrients are taken up by the plant in the following forms: nitrate-nitrogen (NO3--N), sulfate-sulfur (SO42- - S), boric acid (H3BO3) and borate ions (BO33- - B), and chlorine is taken up as the chloride ion (Cl-).

Figure 1. The root system sorption zone and an illustration of the large volume of soil
from which plants extract mobile nutrients.
In a crop field where many plants are growing together, there are commonly root system sorption zones commonly overlap. Therefore, the root system sorption zones for adjoining plants are competing for water and mobile nutrients, (Figure 2). This is one of the main reasons that appropriate plant populations are important for optimum yield.
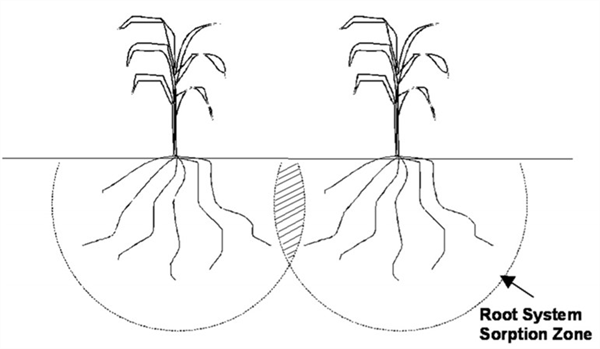
Figure 2. Competition among plants brought about by increasing yield goal.
Immobile Nutrients
Plant nutrients that are immobile in the soil include phosphorus(P), potassium (K), calcium (Ca), magnesium (Mg), iron (Fe), zinc (Zn), manganese (Mn), copper (Cu), and molybdenum (Mo). Immobile nutrients do not move as freely in the soil solution as the mobile nutrients do. These nutrients interact more directly with soil colloids and root surfaces.
Immobile nutrients are absorbed by the plant from the soil and soil solution that is directly next to the root surface. Plant roots must grow through the soil volume to come into direct contact with the immobile nutrients.
Thus, only a small volume of soil and soil solution that immediately adjacent to the root surface will be involved in providing immobile nutrients to the plant. Figure 3 describes this soil volume and plant root interface as the root surface sorption zone.

Figure 3. The root surface sorption zone and an illustration of the small volume of soil
from which plants extract immobile nutrients.
In the case of immobile nutrients, the entire soil volume is not as important as the soil colloid surfaces and soil solution next to the root surface. The concentration of immobile nutrients on the soil colloids immediately next to the root surface is a critical of the root system sorption zone.
Since only a thin layer of soil surrounding and in direct contact with the plant roots are involved in supplying immobile nutrients to the plant, there is little or no competition among plants for immobile nutrients. Competition among plants only occurs at points where roots from adjacent plants come in direct contact with one another (Figure 4).
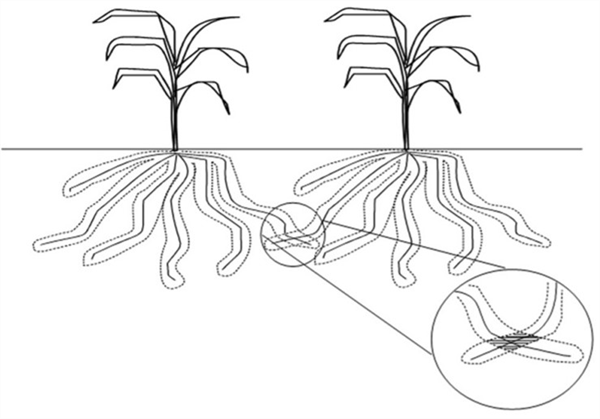
Figure 4. Limited competition among plants for immobile nutrients.
Due to this manner of immobile nutrient behavior and interaction with plant roots, the supply or concentration of immobile nutrients such as phosphorus (P) or potassium (K), is not dependent on a yield goal. In the case of immobile nutrients, the overall soil concentration of the immobile nutrients is most important, and these nutrients are not moving readily with soil water. If the immobile nutrient supply in the soil is adequate for optimum yield of a crop, the healthy plant root system can explore new soil volume and extract the nutrient sufficiently, such as phosphorus (P) or potassium (K).
The nutrient mobility concept and these basic illustrations can help us understand the basis for some common observations and resultant crop management practices. Fertilizers with immobile plant nutrients are more effective when they are incorporated into soil and particularly in soil zones where there is a high probability of plant roots encountering the immobile nutrients.
Banded applications of immobile nutrients are generally more effective than the same rates broadcast and incorporated into the soil. In contrast, mobile nutrients like nitrogen (N) can be broadcast and moved into the root system sorption zone by water.
Soil tests for immobile nutrients do not normally change much from year to year and this is true irrespective of the crop yields from the previous season or fertilizer rate. This is because most of soil volume was not in direct contact with the plant roots. Soil concentrations of immobile nutrients do not usually change rapidly but they can be slowly mined out of the soil by a series of crops without proper fertilization.
Continued or over-applications of immobile nutrient fertilizers, such as phosphorus (P), will cause a buildup of that nutrient in the soil. This is because only a small fraction (commonly 15-20% for most crops) of the nutrient or fertilizer comes into direct contact with the plant roots. The remaining amount of fertilizer interacts with the soil.
Appropriate soil tests that are properly correlated and calibrated with crop-specific response categories are important in evaluating immobile plant nutrient status. Immobile nutrient levels in the soil are commonly expressed in terms of percent sufficiency to produce a specific crop based on appropriate soil test results.
Our goal in plant nutrition management is to achieve the highest levels of efficiency (agronomically, economically, and environmentally) in the field as possible
References:
Bray, R.H.1954. A Nutrient Mobility Concept of soil-plant relationships. Soil Sci. 78(1), p. 9-22.
Havlin, J.L., Beaton, J.D., Tisdale, S.L. and Nelson, W.L. 2014. Soil Fertility and Fertilizers; An Introduction to Nutrient Management. 6th Edition, Prentice Hall, Upper Saddle River, NJ.
Silvertooth, J.C. 2024. Soil Health - Bray’s Nutrient Mobility Concept and Mobile Plant Nutrients University of Arizona Vegetable IPM Newsletter, Volume 15, No. 24,
Raun, W.R. 2017. In: Warren et al. 2017. Oklahoma Soil Fertility Handbook, Id:E-1039
Troeh, F.R. and Thompson, L.M. (2005) Soils and Soil Fertility. Sixth Edition, Blackwell, Ames, Iowa, 489.
Warren, J., H. Zhang, B. Arnall, J. Bushong, B. Raun, C. Penn, and J. Abit. 2017. Oklahoma Soil Fertility Handbook. Id: E-1039
Weil, R.R. and Brady, N.C. (2017) The Nature and Properties of Soils. 15th Edition, Pearson, New York.
At events and in the halls of the Yuma Agricultural Center, I’ve been hearing murmurings predicting a wet winter this year…
As the Yuma Sun reported last week, “The storms of Monday, Aug. 25 [2025], were the severest conditions of monsoon season so far this year in Yuma County, bringing record-rainfall, widespread power outages and--in the fields--disruptions in planting schedules.”
While the Climate Prediction Center of the National Weather Service maintains its prediction of below average rainfall this fall and winter as a whole, the NWS is saying this week will bring several chances of scattered storms.
These unusually wet conditions at germination can favor seedling disease development. Please be on the lookout for seedling disease in all crops as we begin the fall planting season. Most often the many fungal and oomycete pathogens that cause seedling disease strike before or soon after seedlings emerge, causing what we call damping-off. These common soilborne diseases can quickly kill germinating seeds and young plants and leave stands looking patchy or empty. Early symptoms include poor germination, water-soaked or severely discolored lesions near the soil line, and sudden seedling collapse followed by desiccation.
It is important to note that oomycete and fungal pathogens typically cannot be controlled by the same fungicidal mode of action. That is why an accurate diagnosis is critical before considering treatments with fungicides. If you suspect you have seedling diseases in your field, please submit samples to the Yuma Plant Health Clinic or schedule a field visit with me.
National Weather Service Climate Prediction Center: https://www.cpc.ncep.noaa.gov/
National Weather Service forecast: https://forecast.weather.govI thought I’d change pace this week and take the opportunity to ask you, the readers of this newsletter, if there are any ag automation/mechanization topics you would be interested in hearing more about. I am happy to do some research and share the findings in future articles. Drop me a line at siemens@cals.arizona.edu or feel free to call me at 928.782.5869. I’d love to know your interests.

Fig. 1. Futuristic farming.
(Image credits: AgTech Media Group, Dover, DE)
One commonly used product has been Roundup, and the label recommendations are the following: “This product provides weed control when applied prior to harvest of feed barley and wheat. For feed barley, apply after the hard-dough stage and when the grain contains 20 percent moisture or less. For wheat, apply after the hard-dough stage of grain at 30 percent or less grain moisture” 3.
There are concerns of the presence of glyphosate in shipments from US and Canada which they attribute to “the practice of pre-harvest spraying”. Therefore, some growers are looking for alternatives to this herbicide2.
Sharpen (saflufenacil) is a quick acting burndown product for broadleaf weed control in field crops and it has been labeled and approved in the US for this purpose.
Consulting with a BASF representative we learned that Sharpen is labeled in Arizona (not CA) for this use. The recommended rate is 1-2 fl oz., adding Ammonium Sulfate adjuvant and Methylated Seed Oil. Also, apply at the hard dough stage with no more than 30% moisture, with 10 GPA by ground or 5 GPA by air.
Knezevic (2009) stated “MSO was the adjuvant that provided the greatest enhancement of saflufenacil across all species tested. COC was the second-best adjuvant and provided control similar to MSO on many weed species. NIS provided the least enhancement of saflufenacil4.
An Agronomy Update from Kansas State University recommended to “Consult grain buyer to see if they will accept Sharpen treated wheat because of export restrictions”5.

References:
1. http://canola.okstate.edu/cropproduction/herbicides/labels/Roundup%20PowerMax%20Label.pdf
2. https://sustainablepulse.com/2019/08/29/unique-hair-testing-project-reveals-high-levels-of-glyphosate-and-ampa-in-members-of-the-japanese-parliament/
3. https://www.farmprogress.com/weeds/controlling-broadleaf-weeds-in-winter-wheat-is-essential
4. Knezevic, S. Z., Datta, A., Scott, J., & Charvat, L. D. (2009). Adjuvants influenced saflufenacil efficacy on fall-emerging weeds. Weed Technology, 23(3), 340-345.
5. https://cropwatch.unl.edu/2019/ksu-pre-harvest-weed-control-wheat-sharpen-update
Researchers at the University of Wisconsin-Madison have developed a device called “Insect Eavesdropper” that allows real-time detection of insect pests attacking crops. The Insect Eavesdropper is equipped with highly sensitive contact microphones which allow it to capture the sounds of insects eating and moving inside plants. The researchers claimed that by eavesdropping on the sounds within the plants, one can gain insights into insect presence, insect density, insect interaction with the crops, insect feeding behaviors, etc. The device works by clipping a sensor onto plants in the field while the data is monitored remotely to determine when pests are active and where they are concentrated. According to the inventors, by using advanced algorithms, the device can accurately identify the type of pest present, even before visual signs of infestation become apparent.
If this device works as described, it would be a very useful tool for pest monitoring. I you would like to read more about the Insect Eavesdropper technology click on this link.

Results of pheromone and sticky trap catches can be viewed here.
Corn earworm: CEW moth counts down in most over the last month, but increased activity in Wellton and Tacna in the past week; above average for this time of season.
Beet armyworm: Moth trap counts increased in most areas, above average for this time of the year.
Cabbage looper: Moths remain in all traps in the past 2 weeks, and average for this time of the season.
Diamondback moth: Adults decreased to all locations but still remain active in Wellton and the N. Yuma Valley. Overall, below average for January.
Whitefly: Adult movement remains low in all areas, consistent with previous years.
Thrips: Thrips adults movement decreased in past 2 weeks, overall activity below average for January.
Aphids: Winged aphids are still actively moving, but lower in most areas. About average for January.
Leafminers: Adult activity down in most locations, below average for this time of season.