
The cycling of nitrogen (N) in the soil is one of the most important aspects of soil health. Nitrogen cycling in the soil includes a multitude of interactions with microbes and plants.
The basics of soil N were described in a recent article posted in this UA Vegetable IPM newsletter (Silvertooth, 2024). The overall N reactions in the soil system are described in the N Cycle, Figure 1.
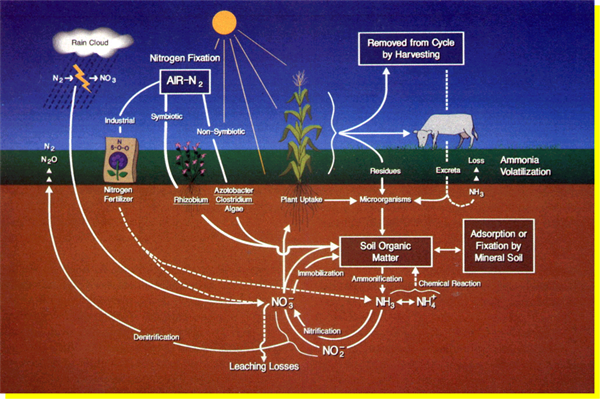
Figure 1. The nitrogen cycle.
Most of the N in soil is present as organic N in the soil organic matter. As a general estimate, there are approximately 1,000 pounds N per acre of this form for every one percent organic matter in the soil. However, soil organic matter (SOM) is relatively stable and resistant to further decay. So, most of the organic N in the soil is not biologically available during any given growing season (Havlin, et al. 2014;Thompson and Troeh, 2005; Warren, et al., 2017; and Weil and Brady, 2017).
Normally, about two percent of the N from SOM will be released each year to mineral forms when soil is cultivated. Arizona soils typically have approximately 1%organic matter. Thus, 10 to 20 pounds of N per acre is a typical amount of N present in unfertilized soils after cultivation and seed bed preparation.
We commonly assume that 1 acre-furrow slice of soil (soil that is 6 inches deep covering an area of 1 acre (43,560 ft.2))contains approximately 2 million pounds of soil. Thus, a soil in Arizona with 1% organic matter will have approximately 20,000 lbs. of SOM per acre and that will contain about1,000 lbs. of total N (5% N in the SOM), most of that being in an organic form that is not directly plant-available.
If 2% of the N in the SOM does mineralize in one year, that would release approximately 20 lbs. of N per acre per year. Accordingly, it is common to find 10 to 20 pounds of mineral plant-available N (NO3--N) per acre. That is a typical amount of mineral plant-available N present in unfertilized soils after cultivation and seed bed preparation. Higher levels of residual NO3--N is commonly a remnant of prior fertilizations.
Nitrogen Mineralization and Immobilization
Because N release from SOM is dependent upon decay by microorganisms, which themselves require N, the amount of N available for a crop is in constant flux. Unlike plants, which get their carbon (C) as carbon dioxide (CO2) from the air, many microorganisms get C from decaying organic matter.
Nitrogen availability depends upon the relative amount of C and N in the SOM, its resistance to decay, and the environmental conditions supporting microbial activity. Figure 2 illustrates how mineral N becomes more concentrated in SOM as soil organic matter decays.

Figure 2. The carbon to nitrogen ratio (C:N) declines and narrows as residues decay
until mineral nitrogen finally becomes available.
(Source: B. Raun In: Warren, et al., 2017)
Note that mineral N is not released during the first stages of decay. This is because N that is released is immediately consumed by active microorganisms. With time, remaining SOM becomes more resistant to decay, microorganisms die off, and there is more mineral N present than can be consumed by the few active microorganisms.
The first step of measurable mineral N release from SOM is usually in the form of ammonia NH3. The ammonia readily reacts with soil moisture to form ammonium NH4+. These two reactions can be stated simply as:
1) Organic nitrogen → NH3 (gas)
2) NH3 + H2O → NH4+ + OH- (ammonia + water → ammonium + hydroxide)
The process of converting or transforming N from organic compounds to inorganic compounds is called mineralization. This results in increasing N available for crops. The N form that is directly available to plants is nitrate-N (NO3--N).
When the reverse happens, plant-available N, primarily nitrate-N (NO3--N) is absorbed by crops or microorganisms. This process is called immobilization and results in a decrease in the amount of N in the soil solution that is immediately available for crops.
These processes of mineralization and immobilization and their interacting nature for a typical field soil situation are illustrated in Figure 3 starting with 2,060 lbs. of total soil N in this case partitioned among the SOM, crop residues, and mineral N pools.
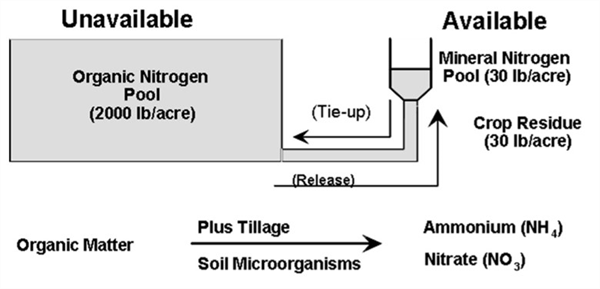
Figure 3. Interacting pools of soil nitrogen.
(Source: B. Raun In: Warren et. al., 2017)
Approximately 98 percent of the soil N is unavailable for plant uptake. This large reservoir of organic N provides an important buffer against rapid changes in available N and plant stress. The small reservoir of mineral N can often be slowly replenished by mineralization (Figure 2.2).
Supplemental N as fertilizer usually must be added to support high, economic crop production levels when native levels of soil N mineralization are not sufficient. This is common in Arizona agricultural soils.
Example Case:
Immediately following fertilization with 120 pounds N, the system may be illustrated by Figure 4a. Note the increase of 120 pounds of N in the mineral N pool in addition to the original 60 lbs. of mineral N in this case (Figure 3), for a total of180 lbs. of mineral N. While the organic N pool is static at 2000 lb./acre in this example. In this case there is a total of 2,180 lbs. Nin the soil.
Addition of fertilizer N will stimulate microorganism activity, resulting in consumption of mineral N by soil microbes as they attack and break down some of the crop residues (immobilization) as illustrated in Figure 4b. Note that the total amount of N among the three pools (mineral, organic, and crop residues) is the same at 2,180lbs. N/acre.
The immobilized N will be present as microbial tissue and other new material in the organic pool. Note that the N present in the crop residue has dropped from 30 to 20 lbs. N/acre in this example due to microbial decomposition.
An important point to follow in this example is the transformations taking place with the consistent base of 2,180 lbs. of N partitioned into various forms. This is a rather simplified example, but it illustrates the natural dynamics taking place in the soil-plant system with the cycling of N and the challenges of managing N fertilizers effectively.
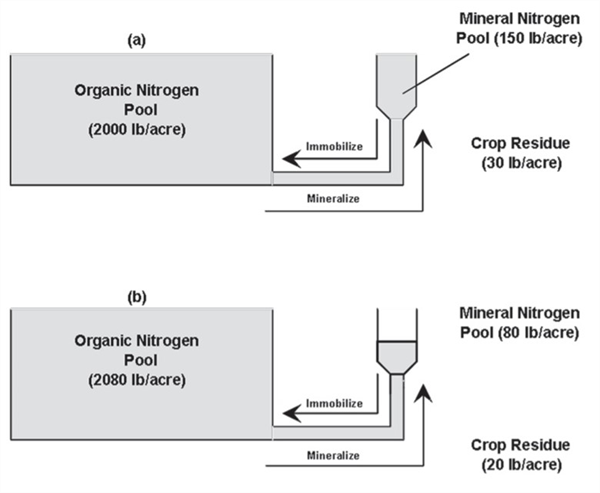
Figure 4. Relative amounts of organic and mineral nitrogen in soil immediately after
fertilizing (a) and several days after active immobilization (b).
As indicated by the two arrows pointing in opposite pathways, mineralization and immobilization are both taking place simultaneously. Immobilized fertilizer N will again become available in a few weeks if conditions favor crop uptake.
This exchange in N forms is often referred to as the mineralization-immobilization transformation (MIT) process and it is a core element of N cycling and management for crop production.
There is a constant flux taking place in soils among these pools of soil N through the MIT process and it is important to be aware of and understand this process when managing fertilizer N inputs for crop production.
References
Havlin, J.L., Beaton, J.D., Tisdale, S.L. and Nelson, W.L. 2014. Soil Fertility and Fertilizers; An Introduction to Nutrient Management. 6thEdition, Prentice Hall, Upper Saddle River, NJ.
Silvertooth, J.C. 2024. Soil Health – Nitrogen. University of Arizona Vegetable IPM Newsletter, Volume 15, No. 22,
Troeh, F.R. and Thompson, L.M. (2005) Soils and Soil Fertility. Sixth Edition, Blackwell, Ames, Iowa, 489.
Warren, J., H. Zhang, B. Arnall, J. Bushong, B. Raun, C. Penn, and J. Abit. 2017. Oklahoma Soil Fertility Handbook. Published Apr. 2017; Id: E-1039
Weil, R.R. and Brady, N.C. (2017) The Nature and Properties of Soils. 15thEdition, Pearson, New York.
I hope you are frolicking in the fields of wildflowers picking the prettiest bugs.
I was scheduled to interview for plant pathologist position at Yuma on October 18, 2019. Few weeks before that date, I emailed Dr. Palumbo asking about the agriculture system in Yuma and what will be expected of me. He sent me every information that one can think of, which at the time I thought oh how nice!
When I started the position here and saw how much he does and how much busy he stays, I was eternally grateful of the time he took to provide me all the information, especially to someone he did not know at all.
Fast forward to first month at my job someone told me that the community wants me to be the Palumbo of Plant Pathology and I remember thinking what a big thing to ask..
He was my next-door mentor, and I would stop by with questions all the time especially after passing of my predecessor Dr. Matheron. Dr. Palumbo was always there to answer any question, gave me that little boost I needed, a little courage to write that email I needed to write, a rigid answer to stand my ground if needed. And not to mention the plant diagnosis. When the submitted samples did not look like a pathogen, taking samples to his office where he would look for insects with his little handheld lenses was one of my favorite times.
I also got to work with him in couple of projects, and he would tell me “call me John”. Uhh no, that was never going to happen.. until my last interaction with him, I would fluster when I talked to him, I would get nervous to have one of my idols listening to ME? Most times, I would forget what I was going to ask but at the same time be incredibly flabbergasted by the fact that I get to work next to this legend of a man, and get his opinions about pest management. Though I really did not like giving talks after him, as honestly, I would have nothing to offer after he has talked. Every time he waved at me in a meeting, I would blush and keep smiling for minutes, and I always knew I will forever be a fangirl..
Until we meet again.
At the UC Cooperative Extension 2024 Automated Technology Field Day in Salinas, CA a couple of weeks ago, 12 of the latest automated and robotic technologies were demonstrated in the field. Most were designed for weed control or thinning vegetable crops. Several of the technologies shown are relatively “new” for the 2024 season. These included laser weeders that also are capable of thinning lettuce (Fig. 1 & 2), a smart cultivator/side-dresser that cultivates between individual crop plants and simultaneously applies fertilizer at a variable rate depending on plant size (Fig. 3), a spot sprayer that utilizes superheated vegetable oil to kill weeds (Fig. 4), and a self-propelled machine that disinfests soil prior to planting using steam. Although the test runs were short, I was impressed with the possibilities for these machines. Company representatives said they will be in Arizona this upcoming season and are interested in meeting with growers. Company contact information can be found at their respective websites, or feel free to contact me if you would like additional information.

Fig. 1. Carbon Robotics’1 (Seattle, WA) LaserWeederTM. The unit utilizes a vision
system to detect crop plants and weeds. A laser is used to kill unwanted plants.
The machine can be used for weeding or thinning lettuce crops (bottom).

Fig. 2. L&A1 (Chico, WA) autonomous laser weeding/thinning robot. The unit is
equipped with a vision system to detect crop plants and weeds, and a laser to kill
unwanted plants. The machine can be used for crop thinning or weeding (weeded
carrot crop, bottom).

Fig. 3. Stout Industrial Technology’s1 (Salinas, CA) smart cultivator/side
dresser. The unit is equipped with a vision system for detecting crop plants, and
blades that move in and out of the crop row to remove in-row weeds. Liquid
fertilizer (shown colored with blue dye) is applied at a variable rate depending on
plant size.

Fig. 4. Tensorfield Agriculture1 (Union City, CA) precision spot spray weeding
machine. The unit has an 80” wide spray boom equipped with 232 individually
controllable spray nozzles to spot spray weeds with superheated vegetable oil
(bottom left, bottom right). A vision system is used to detect weeds and spot
spray resolution is ¼”.

Fig. 5. UA/UC Davis self-propelled band-steam applicator. Device injects steam
into the soil to kill weed seed and soilborne pathogens prior to planting.
[1] Reference to a product or company is for specific information only and does not endorse or recommend that product or company to the exclusion of others that may be suitable.
We promised to publish your comments on the last article on Napropamide. Your honest opinion is welcomed, and we understand that sometimes your views and experiences will differ from ours.
You have reported that uniformity of broccoli harvesting was affected by the application of Napropamide. Having too many harvesting events can obviously increase the cost of your operations.
Thank you for communicating this to us. According to some authors in crops such as wheat yield can be improved by minimizing plant-to-plant variability in seedling emergence1. We value your perspective as we are all working for the benefit of the industry.
The evaluations conducted at the UA Yuma Agricultural Center were not taken to harvest and therefore no crop maturity data was collected this season. Hopefully will do this in future trials.
Other comments:
“Thanks for the Devrinol trial work. I had almost forgot about this material. We used to use it on Cantaloupe and Tomatoes up here years ago. I recall one experience where a new grower used it under plastic and suffered high plant stunting and death. In most cases in open furrow production, it worked great”. –PCA/Grower
“I reduced the rate to 1pt and worked good”. --PCA
Note on Dacthal
We wanted to share some information on the revision of the registration of DCPA.
On April 1, 2024 the EPA published new information on Dacthal here: EPA publication on DCPA released yesterday. It was a warning that has been posted in various web sites.
Previously on March 27, 2024 response (EPA-HQ-OPP-2011-0373-0112) to the registrant the EPA says the following: “Given our inability to identify and agree on a mitigation strategy that addresses EPA’s concerns, the agency is currently exploring the regulatory options available to it under FIFRA Section 6. Due to the serious potential risks posed by DCPA use, EPA will be pursuing these regulatory options as soon as practicable.” Therefore, we are awaiting final decision.
Some members the industry are expecting this herbicide to be available this season. The Vegetable IPM Team encourages you to check the latest information following the link :https://www.regulations.gov/docket/EPA-HQ-OPP-2011-0374/document?postedDateFrom=2024-04-01&postedDateTo=2024-04-03
Thank you very much for your comments they are very useful and beneficial for the industry.
References
Resistant varieties are crucial for effective insect pest management, more specifically in organic crop production. When growing crops organically, resistant varieties should be the first line of defense against insect pests. Compared to nonresistant varieties, insect-resistant crop varieties are less vulnerable to insect attacks or yield well despite insect attacks.
Resistance in crops can be expressed through different mechanisms, including antibiosis, antixenosis, and tolerance. For the antibiosis mechanism, the resistant crops act on the life history traits of the pest, such as affecting fecundity, growth, development, and survival. Thus, this reduces the pest population. On the other hand, antixenosis is characterized by the ability of the crop to affect the behavior of the pest by deterring it after initial tasting or probing, which is non-preference. For the tolerance mechanism, however, the crops can sustain a high density of pests and still maintain their yield potential. In most cases, the overall resistance of a variety is a combination of all these three mechanisms.
There is also a type of resistance called phenological resistance, field resistance, or pseudo-resistance, which is when the crop appears to be resistant because it is at a less susceptible stage when a pest attack occurs. This may happen either because of the rate of development of the crop or due to management practices implemented, causing the crop to escape the pest pressure. Thus, the implementation of proper agronomic practices plays a significant role in improving the ability of crops to resist pest attacks.
In certain scenarios, additional insect control measures such as the use of bioinsecticides (in organic production) and natural enemies may be necessary in conjunction with an insect-resistant variety for comprehensive pest control. However, even in cases where additional control measures are required, the cultivation of a resistant variety can significantly delay and reduce the amount of insecticide applications needed. This underscores the economic, ecological, and environmental benefits of using insect-resistant crop varieties.
Previous studies conducted at Yuma Agricultural Center demonstrated that resistant lettuce varieties can provide suitable control of aphids affecting lettuce in the region. Therefore, it is very important to consider cultivating insect-resistant lettuce varieties for suitable management of aphids in organic lettuce production since biological insecticide options are currently limited. My lab is planning to evaluate several lettuce varieties to determine lettuce varieties that carry resistance against aphids in an attempt to generate applicable information on varieties that could fit well within the production practices and environmental conditions of Arizona’s vegetable-growing regions.