Aug 7, 2024
American Perceptions on Personal Water Requirements
For those of us working in agriculture, we develop an appreciation for how much water is required to produce crops. The average American citizen does not really think about water requirements for food or anything we use daily beyond what we directly consume or pour out of the faucet.
The real dangers in not having a good understanding of individual water requirements on a personal level are the impacts on water policy and management. This is certainly true in a desert environment and particularly during times of drought and water shortages.
Every day in the United States every person generally consumes about 100 gallons of water to support our basic needs for drinking, bathing, cooking, toiletries, etc. (USGS, 2019; Kobir, 2024; and Philadelphia City Government, 2024). The United States Geological Survey (USGS) estimates that the average American needs 80-100 gallons per day for basic use and consumption (indoor use). The Arizona Department of Water Resources (ADWR) estimates that Arizonans consume an average of 146 gallons of water per day (ADWR, 2019 and 2024).
As discussed in recent articles in this newsletter (Silvertooth, 2024a and b) the water required to produce the food supporting an average Arizonan can range from 800 to 1,500 gallons per day, depending on a person’s diet. Thus, we can use an estimate of 1,000 gallons consumed per day per person to support our basic food requirements (Anyabwile and Walker, 2019; Wheeler, 2022; Food Print, 2024; Michel, 2023; Smith, 2012).
If this estimate of “virtual water” use is expanded to include clothing, appliances, vehicles, and other items in our common daily use, the average water footprint for Americans easily comes up to 2,000 gallons of water per day (ASPE, 2022).
Considering daily indoor use and diet, a person can develop estimates on their own personal daily water consumption and water footprint by use of one of the water footprint calculators available on-line (i.e., Water Footprint Calculator).
Some recent surveys have been conducted to measure the perceptions of American citizens regarding the water required to produce our food (ASPE, 2022 and Martin, 2021). The results reveal a significant gap between the reality of the water required to produce a person’s food and other basic items regularly used to support daily life. For example:
- Almost 90 million Americans believe it takes no water at all to make a pair of jeans. In fact, a fresh pair of jeans requires around 2,600 gallons to make.
- It takes 713 gallons of water to make a new cotton t-shirt to pair with those jeans. Americans think it just takes 136 gallons.
- Americans believe it takes 158 gallons of water to produce a smartphone, whereas it is more than 3,400.
- Men, on average, estimate higher water consumption than women,140 gallons vs. 60 gallons, but neither group estimated anywhere close to the American’s calculated water footprint of 2,000 gallons per day.
- Gen Z respondents, ages 18–24, had the closest estimate at 365 gallons; however, this is still more than 1,500 gallons lower than the actual amount. The average estimate drops off steeply for Millennials, ages 25–40, at 36 gallons and American citizen estimates remain low throughout older generations as well.
- 77 percent of Boomers, ages 57 and up, stop to appreciate access to clean drinking water fairly often, with 48 percent of Gen Z respondents reporting they do the same. This is based on a national survey but hopefully that is higher in places like Arizona.
- Homeowners estimate their daily water consumption at nearly 130 gallons, compared to renters who believe they use only 33 gallons of water each day. Both are extremely low estimates.
- The actual water consumption for homeowners and renters, based on a national average, is 1,188 and 818 gallons, respectively.
One survey was conducted in the fall and respondents were asked to estimate how much water is required to produce some common food items consumed at Thanksgiving. Some of those results include:
- One 16-pound holiday turkey takes 4,688 gallons vs. the citizen estimated 158 gallons.
- A pecan pie takes 1,068 gallons vs. the citizen estimated 135 gallons; and a pumpkin pie takes 458 gallons vs. the estimated 135 gallons.
- The traditional green bean casserole – with fried onions on top! – takes 547 gallons of water to hit the holiday dinner table vs. the estimated 116 gallons.
With the importance of electronics in our world and daily operations, we can also consider the water requirements for microchip production. When creating an integrated circuit on a 300mm wafer, approximately 2,200 gallons of water are required, including 1,500 gallons of ultrapure water. Consequently, a single large semiconductor fabrication facility processing around 40,000 wafers monthly could consume up to 4.8 million gallons of water daily (approximately 15 acre-feet per day or about 4,000 acre-feet per year). This is equivalent to the annual water use of a city of 60,000 residents (CWR, 2013).
Water is essential to support all life and it takes a lot of water to grow plants and support animals that everyone needs to live and survive. The important point to consider is that we all use more water every day than is commonly realized.
Some responses to this may be “Who cares what people think and what difference does it make?” The problem is that water policy is determined largely by politicians who are often
not fully informed, and they are driven by voters who are commonly ignorant of the facts. This can be a real problem when politicians enact policies based on perceptions and feelings that affect agricultural water allocations.
As the saying goes,
“Facts may be facts, but perceptions become people’s reality.” That is certainly true, and perceptions often only change when confronted with harsh realities. We all have a significant water footprint with everything consumed and the more people understand that the better water management decisions we can make.
References:
Anyabwile, A. and S. Walker. 2019. 5 Ways to put food on a water diet. World Resources Institute.
https://www.wri.org/insights/5-ways-put-food-water-diet
Arizona Department of Water Resources. 2019. Water Your Facts. Arizona Water Facts.
https://www.arizonawaterfacts.com/water-your-facts
Arizona Department of Water Resources (ADWR). 2024. Conservation.
https://www.azwater.gov/conservation/public-resources
ASPE. 2022. New research shows most Americans are unaware of their daily water consumption. ASPE Pipeline.
https://aspe.org/pipeline/new-research-shows-most-americans-are-unaware-of-their-daily-water-consumption/#:~:text=Most%20believe%20they%20use%20less%20than%20100%20gallons,indirectly%20%28e.g.%2C%20the%20water%20required%20to%20produce%20food%29.
China Water Risk (CWR). 2013. 8 Things You Should Know About Water & Semiconductors China Water Risk, 11 July 2013.
Food Print. 2024. The Water Footprint of Food.
https://foodprint.org/issues/the-water-footprint-of-food/
Kobir. 2024. How many gallons of fresh water do we use per day.
https://medium.com/rocklinca/how-many-gallons-of-freshwater-do-we-use-per-day-7987edf6b1bb
Martin, L. 2021. New Research Shows Most Americans are Unaware of Their Daily Water Consumption. Business Wire.
https://www.businesswire.com/news/home/20211021005669/en/New-Research-Shows-Most-Americans-are-Unaware-of-Their-Daily-Water-Consumption
Michel, D. 2023. Water and Food: How, When, and Why Water Imperils Global Food Security. Center for Strategic and International Studies.
https://www.csis.org/analysis/water-and-food-how-when-and-why-water-imperils-global-food-security
Philadelphia City Government. 2024. Gallons Used Per Person Per Day.
https://water.phila.gov/pool/files/home-water-use-ig5.pdf
Silvertooth, J. 2024a. Arizona Water Footprint. University of Arizona Vegetable IPM Newsletter. 15 May 2024, University of Arizona No. 10
Silvertooth, J. 2024b. Water Requirements for the Production of Common Food Products, University of Arizona Vegetable IPM Newsletter, 29 May 2024, No. 11
Smith, T. 2012. World Water Day: How much water do you use in a day? Climate Home News.
https://www.climatechangenews.com/2012/03/22/world-water-day-how-much-water-do-you-use-in-a-day/
United States Geological Survey. 2019. How much water do I use at home each day?
https://www.usgs.gov/special-topics/water-science-school/science/water-qa-how-much-water-do-i-use-home-each-day
Water Footprint Calculator. 2024.
https://watercalculator.org/water-footprint-of-food-guide/
Wheeler, M. 2022. Did You Know’ Series: How Much Water Are We Actually Using? Virginia Water Resources Research Center, Virginia Tech University.
https://www.vwrrc.vt.edu/2022/03/31/did-you-know-series-how-much-water-are-we-actually-using/
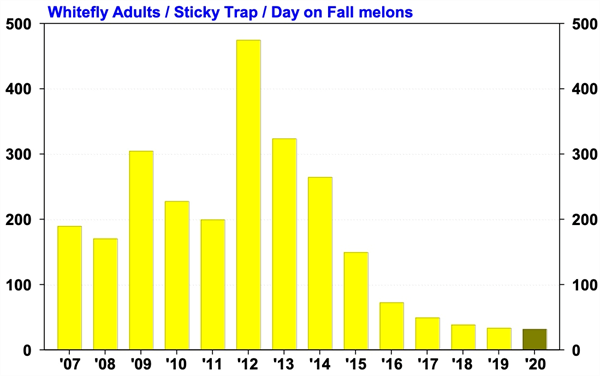
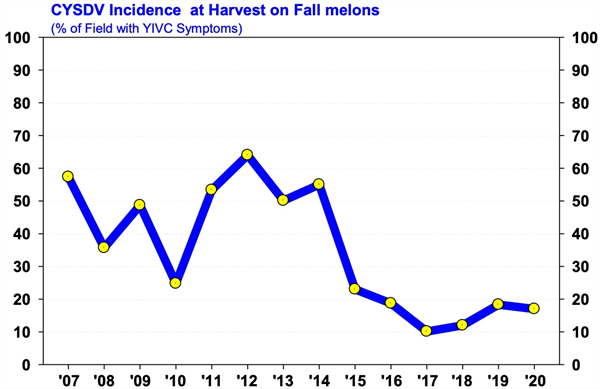 To contact John Palumbo go to: jpalumbo@ag.Arizona.edu
To contact John Palumbo go to: jpalumbo@ag.Arizona.edu






