
Springtime in the desert serves host to a new spring melon/cantaloupe crop. That is particularly true in the lower Colorado River valleys. Cantaloupes (Cucumis melo ‘reticulatus’ L.) or “melons” are one of the important spring and fall vegetable crops of Arizona and the desert Southwest.
Technically, “true” cantaloupes (Cucumis melo ‘cantaloupensis’) are rough, warty fruit, primarily grown in Europe. On a production scale, cantaloupes are not grown commercially in the United States. However, in the United States “cantaloupe” has become a general name of all netted, musk-scented melons (Simonne et al., 1998 and Soto, 2012).
Being able to accurately describe and predict important stages of crop growth and development (crop phenology) and harvest dates is important for improving melon crop management (e.g. fertilization, irrigation, harvest scheduling, pest management activities, labor, and machinery management, etc.). Since plants operate on “thermal time”, they have no regard for calendars or time as we commonly measure it. So, it is best to monitor and predict plant development based on the actual thermal conditions in the plant’s environment.
Various forms of temperature measurements and units commonly referred to as heat units (HU), growing degree units (GDU), or growing degree days (GDD) have been utilized in numerous studies to predict phenological events for many crop plants (Baskerville and Emin, 1969; Brown, 1989; Baker and Reddy, 2001; and Soto, 2012). A graphical depiction of HU computation using the single sine curve procedure is presented in Figure 1 (Brown, 1989).
Since 2000, we have been working on the development and annual testing of a phenology model for desert cantaloupe production for Arizona conditions. The basic cantaloupe phenology model is shown in Figure 2 (Silvertooth, 2003; Soto et al., 2006; and Soto, 2012). This melon crop phenology model was developed under fully irrigated conditions.
Guideposts indicated in Figure 2 represent general average or “target” values and a slight degree of variation is normal. It is important to note that water or nitrogen stress are two factors that will significantly alter physiological development of a crop.
Referencing the data from the Arizona Meteorological Network (AZMET) and several locations in the Yuma area, the HU accumulations (86/55 ºF thresholds) from a range of possible 2024 melon planting dates to 28 April are listed in Table 1. These HU accumulations can be referenced against the melon phenology model in Figure 2 and crop conditions in the field.
I encourage those who are working with spring cantaloupe production this season to test and evaluate this crop phenology model in the field under various planting dates, varieties, and conditions. The information in Table 1 can help serve as a reference to check melon crop development in the field against this phenological model. We appreciate your feedback.
References:
Baker, J.T., and V.R. Reddy. 2001. Temperature effects on phenological development and yield of muskmelon. Annals of Botany. 87:605-613.
Baskerville, G.L., and P. Emin. 1969. Rapid estimation of heat accumulation from maximum and minimum temperatures. Ecology 50:514-517.
Brown, P. W. 1989. Heat units. Ariz. Coop. Ext. Bull. 8915. Univ. of Arizona, Tucson, AZ.
Silvertooth, J.C. 2003. Nutrient uptake in irrigated cantaloupes. Annual meeting, ASA-CSSA-SSSA, Denver, CO.
Simonne, A., E. Simonne, R. Boozer, and J. Pitts. 1998. A matter of taste: Consumer preferences studies identify favorite small melon varieties. Highlights of Agricultural Research. 45(2):7-9.
Soto, R. O. 2012. Crop phenology and dry matter accumulation and portioning for irrigated spring cantaloupes in the desert Southwest. Ph.D. Dissertation, Department of Soil, Water and Environmental Science, University of Arizona.
Soto-Ortiz, R., J.C. Silvertooth, and A. Galadima. 2006. Nutrient uptake patterns in irrigated melons (Cucumis melo L.). Annual Meetings, ASA-CSSA-SSSA, Indianapolis, IN.
|
Location |
15 January |
1 February |
15 February |
1 March |
|
Yuma Valley |
994 |
893 |
832 |
680 |
|
Yuma N. Gila |
972 |
856 |
797 |
650 |
|
Yuma South |
961 |
862 |
802 |
655 |
Table 1. Heat unit accumulations (86/55 ºF thresholds) from four possible 2024 planting dates utilizing Arizona Meteorological Network (AZMET) data for each representative site.
Yuma Valley:https://ag.arizona.edu/azmet/02.htm
Yuma South: https://ag.arizona.edu/azmet/36.htm
Yuma North Gila: https://ag.arizona.edu/azmet/14.htm
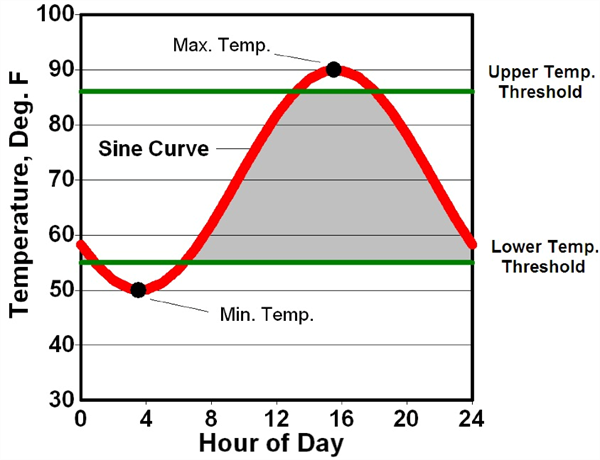
Figure 1. Graphical depiction of heat unit computation using the single sine
curve procedure. A sine curve is fit through the daily maximum and minimum
temperatures to recreate the daily temperature cycle. The upper and lower
temperature thresholds for growth and development are then superimposed
on the figure. Mathematical integration is then used to measure the area
bounded by the sine cure and the two temperature thresholds (grey area).
(Brown, 1989)
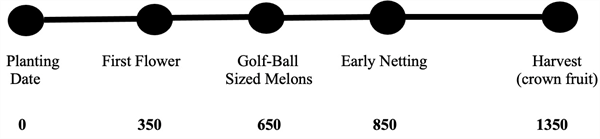
Figure 2. Heat Units Accumulated After Planting (HUAP, 86/55 oF)
Hi, I’m Chris, and I’m thrilled to be stepping into the role of extension associate for plant pathology through The University of Arizona Cooperative Extension in Yuma County. I recently earned my Ph.D. in plant pathology from Purdue University in Indiana where my research focused on soybean seedling disease caused by Fusarium and Pythium. There, I discovered and characterized some of the first genetic resources available for improving innate host resistance and genetic control to two major pathogens causing this disease in soybean across the Midwest.
I was originally born and raised in Phoenix, so coming back to Arizona and getting the chance to apply my education while helping the community I was shaped by is a dream come true. I have a passion for plant disease research, especially when it comes to exploring how plant-pathogen interactions and genetics can be used to develop practical, empirically based disease control strategies. Let’s face it, fungicide resistance continues to emerge, yesterday’s resistant varieties grow more vulnerable every season, and the battle against plant pathogens in our fields is ongoing. But I firmly believe that when the enemy evolves, so can we.
To that end I am proud to be establishing my research program in Yuma where I will remain dedicated to improving the agricultural community’s disease management options and tackling crop health challenges. I am based out of the Yuma Agricultural Center and will continue to run the plant health diagnostic clinic located there.
Please drop off or send disease samples for diagnosis to:
Yuma Plant Health Clinic
6425 W 8th Street
Yuma, AZ 85364
If you are shipping samples, please remember to include the USDA APHIS permit for moving plant samples.
You can contact me at:
Email: cdetranaltes@arizona.edu
Cell: 602-689-7328
Office: 928-782-5879
A couple years ago, we conducted evaluations of various “new” technologies for cultivating weeds in cotton as compared to conventional methods. The new technologies included 1) a camera-guided side-shift hitch and 2) finger weeders, an in-row weeding tool (Fig. 1). Camera-guidance of the maneuverable hitch allows cultivating tools to be positioned close to the seed row. In the study, the uncultivated band was 3.5" for the camera-guided system, and 6” for the conventional cultivator. The aim of evaluating these technologies was to determine their efficacy in controlling herbicide resistant weeds. Trials conducted over 3 years showed that use of camera-guidance improved weed control by more than 30% and finger weeders removed about 45% of the in-row weeds. Overall weed control using the two technologies together was roughly > 90% for broadleaf weeds and about 85% for all weeds species.
Studies conducted by Texas A&M over two years showed similar results (Dotray et. al, 2021).
It is logical to think that similar type results would be realized in vegetable crops such as broccoli and cauliflower, plants that also have fairly long plant stems at the seedling stage of growth. A better than 40% reduction of in-row weeds would significantly lower hand weeding requirements. If you are interested in trying these technologies in vegetable or other crops on your farm, please contact me. We still have the equipment and I’d be happy to work with you.
A presentation given on the trial results and videos of the equipment used operating can be found by clicking here or on image below.
References
Dotray, P.A., Keeling, J.W., & Russell, K.R. 2021. Precision cultivation with finger weeder systems. Project No. 20-190 Final Report. Cary, N.C: Cotton Inc.
Acknowledgements
Project partially funded and supported by Arizona Cotton Growers Association, Cotton Inc., KULT-Kress, LLC and Keithly-Williams Fabrication. We thank them for their support.

Fig. 1. Technologies for precision cultivation and in-row weeding used in
efficacy trials included a a) a camera-guided side-shift hitch attached to a
cultivator and b) in-row weeding tools (finger weeders).
Fig. 2. Click on image above to watch presentation on precision cultivation and
in-row weeding technologies.
Postemergence herbicides used for grass control in vegetables include Poast (sethoxydim), Select (clethodim) and Fusilade (fluazifop) and the generics of these active ingredients. When some grasses have escape or have survived the application of Pefar, Kerb and Balan these selective grass herbicides can come to the rescue and have shown to be a great tool for weed management.
The above-mentioned herbicides are all Acetyl CoA carboxylase or ACCase inhibitors. These products work slowly and even slower at lower temperatures and shorter days. Treated grasses should stop growing immediately and will slowly turn yellow, red and gradually die as the crop grows. The process takes about 15-21days. Select (clethodim) works the faster than Poast (sethoxydim), and it isrecommended to add a crop oil concentrate for better activity with the exception of Select Max which can be used either with crop oil concentrate or non-ionic surfactant.
References:
This time of year, John would often highlight Lepidopteran pests in the field and remind us of the importance of rotating insecticide modes of action. With worm pressure present in local crops, it’s a good time to revisit resistance management practices and ensure we’re protecting the effectiveness of these tools for seasons to come. For detailed guidelines, see Insecticide Resistance Management for Beet Armyworm, Cabbage Looper, and Diamondback Moth in Desert Produce Crops .
VegIPM Update Vol. 16, Num. 20
Oct. 1, 2025
Results of pheromone and sticky trap catches below!!
Corn earworm: CEW moth counts declined across all traps from last collection; average for this time of year.
Beet armyworm: BAW moth increased over the last two weeks; below average for this early produce season.
Cabbage looper: Cabbage looper counts increased in the last two collections; below average for mid-late September.
Diamondback moth: a few DBM moths were caught in the traps; consistent with previous years.
Whitefly: Adult movement decreased in most locations over the last two weeks, about average for this time of year.
Thrips: Thrips adult activity increased over the last two collections, typical for late September.
Aphids: Aphid movement absent so far; anticipate activity to pick up when winds begin blowing from N-NW.
Leafminers: Adult activity increased over the last two weeks, about average for this time of year.







