-
Jul 14, 2021Management Guidelines for Whiteflies and CYSDV on Fall Melons in 2021*
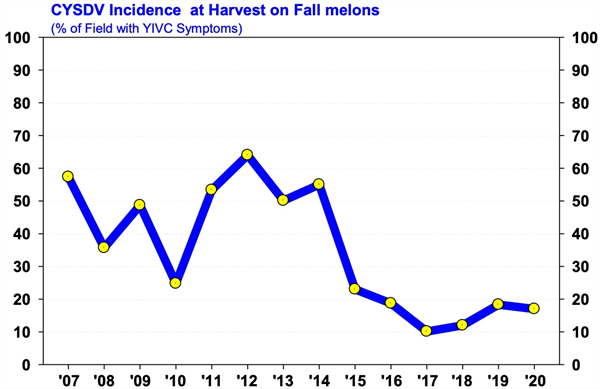
Management Guidelines for Whiteflies and CYSDV on Fall Melons in 2020 After Bemisia whiteflies invaded the desert in 1991, production of fall melons became very difficult, and acreage declined in many desert growing areas. With the Section 18 registration of Admire 2F in 1993, growers could effectively control whiteflies again and soon began to profitably produce fall melons in Yuma, and central Arizona. Then in 2007, a new challenge emerged for melon growers when Cucurbit Yellows Stunting Disorder Virus (CYSDV) became established throughout the desert Southwest. It’s taken some time, but now economic production of fall melons appears to be back on track in Arizona. In the past three growing seasons, whitefly populations during the fall have been lighter than normal, and CYSDV incidence on fall melons was the lowest recorded on melons since the virus was first reported (see graphs below). I believe this is a largely a result of the vigilant whitefly management practices used by PCAs and melon growers. Looking forward, whitefly numbers on melons this spring were moderately higher than we’ve seen the past few years, and CYSDV infected plants could easily be found in most fields still growing in late June and early July. How these low numbers translate into virus incidence on melon crops this fall is unknown? However, given the numbers of whiteflies being found in cotton and alfalfa at the present time, PCAs should be on the lookout for significant whitefly migrations as fall melons begin to emerge in early August. To download the most recent information for whitefly and CYSDV management in fall melons go to:
2021 Guidelines for Whitefly / CYSDV Management on Fall Melons.
The epidemiology of CYSDV is a complex relationship between the virus, the adult whitefly vector and our local melon cropping system. It has been our goal over the past 14 years to understand this pathosystem and develop practical approaches for reducing CYSDV impact on fall melon production. We continue to develop new information on cultural and chemical control tactics for adult whiteflies (see 2021 Guidelines). Our knowledge to date suggests that fall melons produced near cotton, alfalfa or near areas where spring melons were recently produced are at the highest risk of infection. When practicable, growers should attempt to isolate fall melon plantings as far away as possible from these sources of whiteflies and CYSDV. Growers forced to plant fall melons near these crops should be vigilant in minimizing adult whitefly infestation levels with insecticides during pre-bloom growth stages. This can be achieved with at-planting and side-dress applications of soil insecticide (Venom, Scorpion, Sivanto) and well-timed applications of foliar sprays. For Arizona growers, new insecticide labels are available for adult knockdown control. PQZ and Sefina, registered for use on Arizona melons in 2019, are feeding disruptors with a high degree of bee safety. Based on local research, both products will provide excellent adult knockdown and CYSDV suppression when used in a fall management program. Transform (same active ingredient as Sequoia) is also labeled for use on melons and provides another alternative for adult knockdown. Bottom line: cost-effective tools are available but minimizing whitefly abundance in the surrounding crop landscape (e.g., cotton, volunteer melons) is critical for them to be most effective.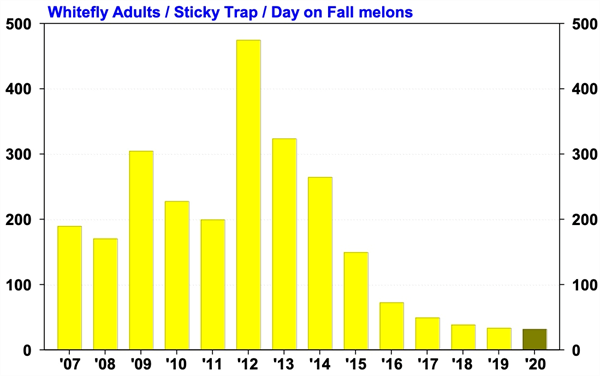
 To contact John Palumbo go to: jpalumbo@ag.Arizona.edu
To contact John Palumbo go to: jpalumbo@ag.Arizona.edu