
I am seeking samples of downy mildew on lettuce from around Yuma County to support the Michelmore Lab and their ongoing efforts to help characterize the downy mildew populations of the United States. The Michelmore Lab has led the charge on a survey of Bremia variants since 1980 and has been instrumental in demystifying the gene-for-gene nature of lettuce resistance to downy mildew.
Their group invites growers across the United States to submit downy mildew infected plant samples, which are then used to culture the Bremia on live host plants. The team then inoculates a panel of lettuce varieties carrying known resistance genes to determine the race of each isolate they receive. Identifying which races occur in which specific fields is essential to guiding the breeding of new resistant cultivars and maximizing the effectiveness of host-based genetic disease management. The data obtained from these tests are also used to designate new Bremia races through the International Bremia Evaluation Board.
Your contribution will help breed better lettuce for Yuma. This means less breakdown of resistance in the field, and better yields for Yuma growers. To facilitate these submissions the Yuma Plant Health Clinic will be setting up a separate drop-off point and submission sheet for downy mildew sample submissions in the same hallway we use for standard plant diagnostic submissions. The drop-off point will be clearly labelled and consist of a chest-style refrigerator and printed copies of the submission form. It is vital to keep these samples cool so they remain viable for future inoculations, so please place your samples inside of the refrigerator before you leave.
Shipping will be handled by the clinic. All we ask is that you fill out the submission form as completely as you can. An example of the questions that are asked in that form so you can prepare ahead of time can be found HERE .It’s October and planting season is well underway. When planting lettuce, uniform seed spacing is critical for efficient, economical crop thinning. Due to their lack of precision, automated lettuce thinning machines cannot eliminate lettuce plants that are spaced closer than about 1 1/8” apart. These closely spaced plants, commonly referred to as “doubles”, must be carefully removed by hand which is time consuming and expensive.
Several years ago, we conducted trials examining the influence of planter travel speed on seed spacing uniformity (Siemens and Gayler, 2016). In the study, two types of planters - a vacuum planter (Stanhay 785 Singulaire) and a belt planter (Stanhay 870) were tested with pelleted lettuce seed at travel speeds of 1.0, 1.5, 2.0 and 2.5 mph at the Yuma Agricultural Center. Vacuum planter test results showed that the percentage of “difficult to thin” spacings, defined seeds spaced less than 1.1” apart, increased from about 5% to 10% as speed increased from 1.0 mph to 2.5 mph (Fig. 1). Concomitantly, the percentage of seeds “precisely placed” within 0.5” of the target location (i.e., 2.0 ± 0.5”) decreased from 70% to less than 45%, and the percentage of skips increased from 7% to 30%. Variability of seed spacing uniformity as measured by the coefficient of variation (COV) of seed spacings also increased.
Similar declines in planter performance were found with the belt planter (Fig. 2). As travel speed increased from 1.0 to 2.5 mph, the percentage of difficult to thin spacings increased from 2% to 10%, precise spacings decreased from 93% to 65% and skips increased from 2% to 8%.
For both planter types, there was a significant decline in performance as speed increased from 1.5 mph to 2.0 mph, a difference in speed of only 0.5 mph. These results suggest that it is prudent to check planter performance at the chosen operating speed prior to establishing an entire block to ensure that seed spacing uniformity, not just the number of seeds per foot, is acceptable.
In short, the study showed that planter travel speed had a significant effect on seed spacing uniformity and difficult to thin, close spacings - the higher the speed, the poorer the performance.
You may be asking what is the reason for the phenomenon observed? A logical explanation is that seeds are traveling at the speed of the planter when they are released and tend to “bounce and roll” in the direction of travel when they hit the soil surface. Thus, the higher the travel speed, the further seeds bounce and roll resulting in increased seed spacing variability.
References
Siemens, M.C., & Gayler, R.R. (2016). Improving seed spacing uniformity of precision vegetable planters. Appl. Eng. Agric., 32(5), 579-587
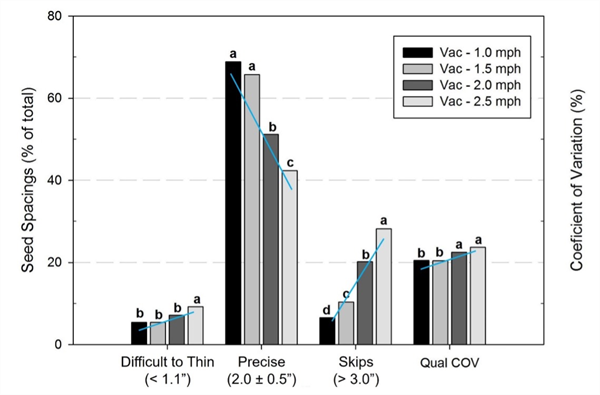
Fig. 1. Seeding performance of a vacuum vegetable planter sowing lettuce when
operated at four travel speeds.

Fig. 2. Seeding performance of a belt vegetable planter sowing lettuce when operated at
four travel speeds
It is reported that the herbicidal activities of Plant Growth Regulators (PGRs) were discovered in the 1940’s. Then, investigators in England and in the United States started their research on this type of herbicides1.
Some of these substances are hormones produced naturally by the plants and other are synthetically produced. Examples of naturally occurring growth regulators are gibberellins, auxin, cytokinin. Some stimulate stem elongation and cell elongation. One of the first synthetic selective herbicides developed is 2, 4-D (2,4-dichlorophenoxiacetic acid).
PGRs are used extensively for broadleaf weed control in grass crops in this region such as grain production, bermudagrass, alfalfa, cole crops, sugarbeet, forages, and turf grasses. These herbicides upset the natural balance of the hormones that controls cell division, cell enlargement, protein synthesis, and respiration. That is why this group of herbicides is sometimes called the “hormone herbicides”2. In our area growers are very careful using these products due to volatility with our summer temperatures and the problems caused to sensitive crops.
According to a report from Texas A&M “phenoxy growth regulator herbicides are reported to have the least plant activity and soil residual activity; the carboxylic acids generally have the most. Broadleaf crops and turf grasses should not be planted into soils recently treated with these herbicides because they severely inhibit seedling emergence”2.
Some PGRs:
|
Family |
Common Name |
Trade Name |
|
phenoxy |
2,4-D |
Pasture pro, others |
|
2,4-DB |
Butyrac |
|
|
MCPA |
Rhonox |
|
|
MCPP |
Several names |
|
|
benzoic acid |
dicamba |
Banvel |
|
carboxylic acid |
Picloram |
Tordon 22K |
|
Clopyralid |
Stinger |
|
|
triclopyr |
Remedy |
|
|
quinclorac |
Facet |