
When we go to the field to evaluate a crop, there are typically three fundamental points we need to take into consideration that include: 1) stage of growth, 2) general crop vigor and yield potential, and 3) anticipate the next stage of development and what we need to do in terms of field crop management. These considerations are commonly focused on aboveground crop evaluations. However, the root system is also an extremely important part of the crop condition to evaluate.
Root systems were described in a basic manner in a recent article on 2 May 2023 (UA Vegetable IPM Newsletter Volume 14, No. 9).
The effective root zone depth is the depth of soil used by the bulk of the plant root system to explore a soil volume and obtain plant-available moisture and plant nutrients. Effective root depth is not the same as the maximum root zone depth. As a rule of thumb, we commonly consider about 70% of the moisture and nutrient uptake by plant roots takes place in the top 24 inches of the root zone; about 20% from the third quarter; and about 10% from the soil in the deepest quarter of the root zone (Figure 1).
The small and very fine root hairs are the most physiologically active portion of a developing root system. It is important that the plants continue to develop and generate fresh young roots and an abundance of fine root hairs to maintain water and nutrient uptake.
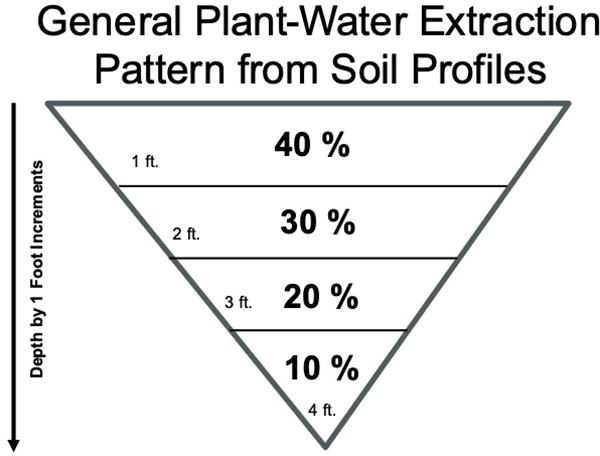
Figure 1. General pattern for plant-water and nutrient uptake from the soil profile.
It is important to point out that these general root development patterns are dependent on the nature of the soil profile in the fields. Soil profiles with compaction layers, as well as rock or caliche layers will limit root development and full exploration of the soil volume that the plants are capable of (Figure 2).
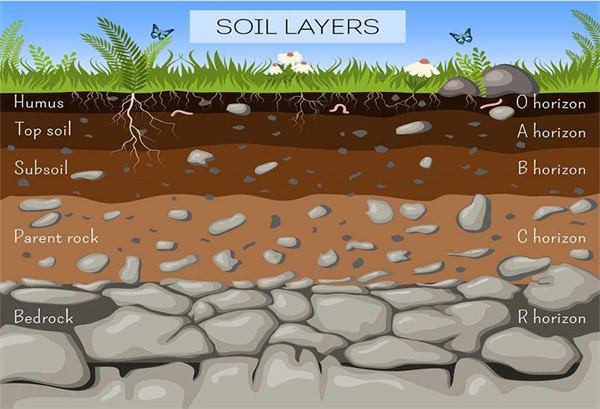
Figure 2. Generalized soil profile with major horizons.
Therefore, in scouting fields and making crop evaluations, examining the root systems is an important part of the process. Leafy green vegetable crops need to develop a marketable plant in a relatively short amount of time and a strong root system is essential.
It does take more time and effort to check root systems and it is a plant destructive process since we need to literally excavate the roots. So, it is also important to be careful of where and how we sample plants and the root systems in a field.
Crop species can vary significantly in their patterns of root development and it is important to know what is “normal” when evaluating crops in the field. An excellent reference for vegetable crop root system development is a 1927 publication by Dr. John E. Weaver and William E. Bruner from the University of Nebraska (Root Development of Vegetable Crops). This publication can be found at the following link:
https://soilandhealth.org/wp-content/uploads/01aglibrary/010137veg.roots/010137toc.html
A few basic examples from the Weaver and Bruner publication are provided in the following figures (Figures 3-10).
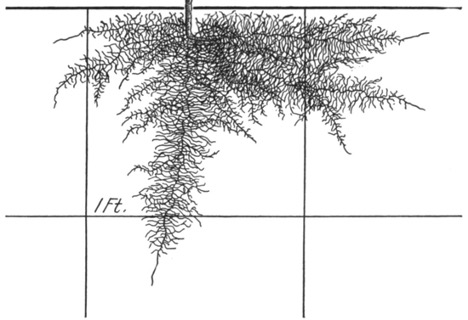
Figure 3. Cauliflower, 3 weeks after transplanting.
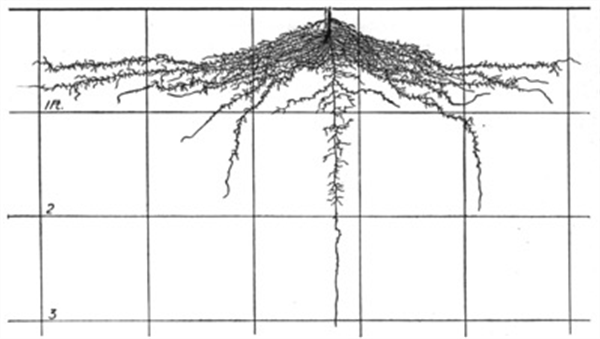
Figure 4. Cabbage roots, 55 days after transplanting.
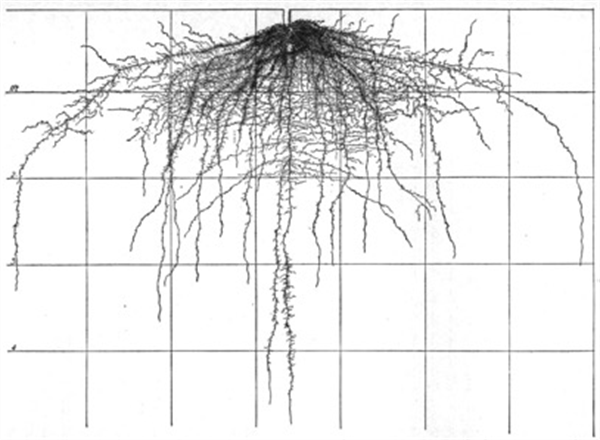
Figure 5. Cabbage roots, 75 days after transplanting.
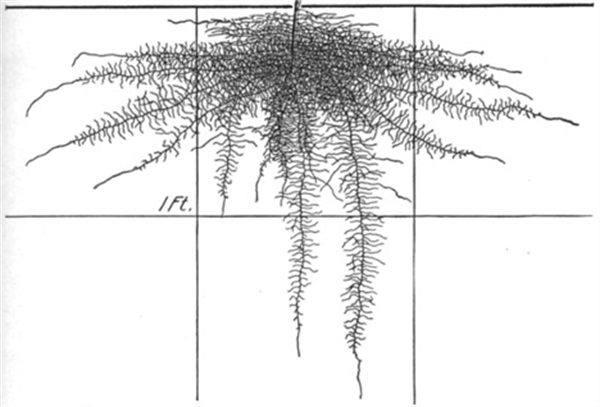
Figure 6. Pepper roots, 24 days.
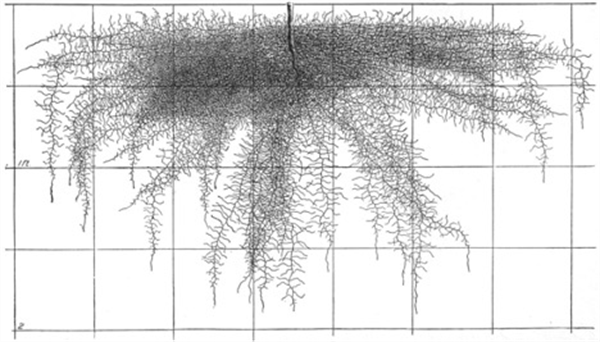
Figure 7. Pepper roots, 45 days (6 weeks).
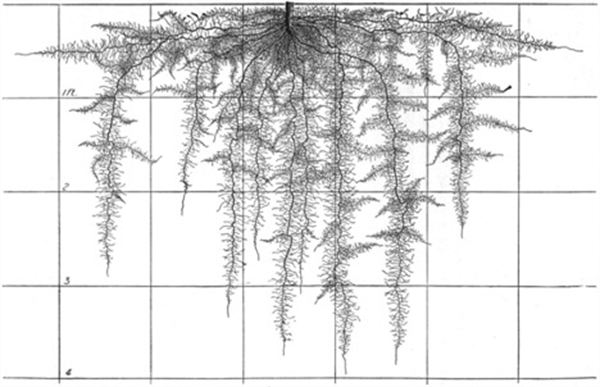
Figure 8. Pepper roots, mature.
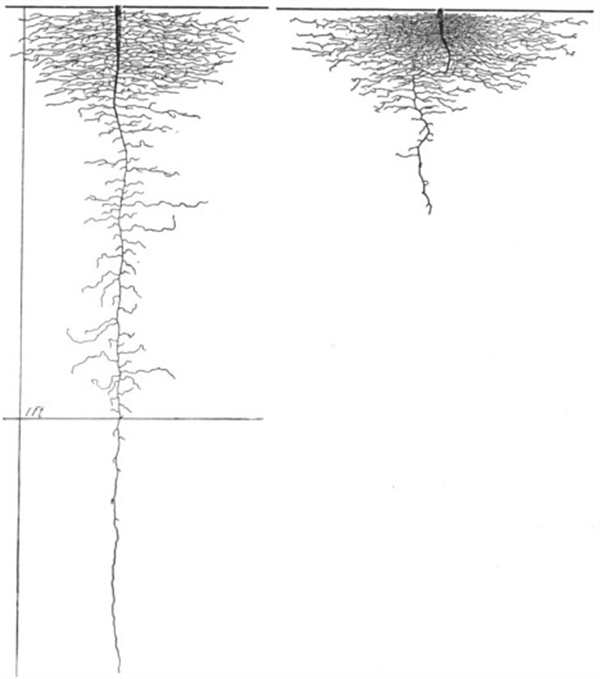
Figure 9. Lettuce roots, 3 weeks. The roots on the right were grown in compact soil, the roots on the
left were grown in loose/open soil.
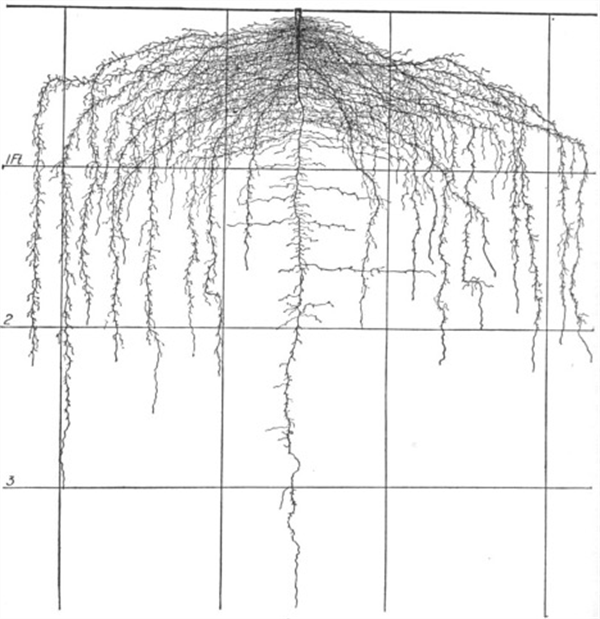
Figure 10. Lettuce roots, 60 days.
Frost and freeze damage affect countless fruit and vegetable growers leading to yield losses and occasionally the loss of the entire crop. Frost damage occurs when the temperature briefly dips below freezing (32°F).With a frost, the water within plant tissue may or may not actually freeze, depending on other conditions. A frost becomes a freeze event when ice forms within and between the cell walls of plant tissue. When this occurs, water expands and can burst cell walls. Symptoms of frost damage on vegetables include brown or blackening of plant tissues, dropping of leaves and flowers, translucent limp leaves, and cracking of the fruit. Symptoms are usually vegetable specific and vary depending on the hardiness of the crop and lowest temperature reached. A lot of times frost injury is followed by secondary infection by bacteria or opportunist fungi confusing with plant disease.
Most susceptible to frost and freezing injury: Asparagus, snap beans, Cucumbers, eggplant, lemons, lettuce, limes, okra, peppers, sweet potato
Moderately susceptible to frost and freezing injury: Broccoli, Carrots, Cauliflower, Celery, Grapefruit, Grapes, Oranges, Parsley, Radish, Spinach, Squash
Least susceptible to frost and freezing injury: Brussels sprouts, Cabbage, Dates, Kale, Kohlrabi, Parsnips, Turnips, Beets
More information:
Based on the recent weather, it’s definitely summer in my book. Things seem to be slowing down, so I thought I’d change pace this week and take the opportunity to ask you, the readers of this newsletter, if there are any ag automation/mechanization topics you would be interested in hearing more about. I am happy to do some research and share the findings in future articles. Drop me a line at siemens@cals.arizona.edu or feel free to call me at 928.782.5869. I’d love to know your interests.

Fig. 1. Futuristic weeding. (Image credits: S.L. Young)
We have seen abundant Shepherdspurse recently in Yuma, AZ. Most of us are familiar with this winter annual broadleaf weed but we are adding a brief description:
Scientific name: Capsella bursa – pastoris
Season: Winter Annual
Habitat: Present in all crops and ditch banks, home gardens.
Cotyledons: Oval with a short petiole.
Leaves: First true leaves with hairs and spoon shape. Can have different shapes and form a rosette soon after emergence.
Young plants: Are mostly basal rosettes.
Mature plants: grow up to 20” with flower stems with sparse small leaves.
Flowers and Fruit: Flowers are pale pink and fruits are heart shaped or triangular seed pods.
Shepherdspurse is from the mustard or crucifer family, which includes many crops grown here such as broccoli, cauliflower, kale, kohlrabi, mustard greens and others.
In our production practices we commonly select some weed species that survive our methods of control. This weed has been around for a long time and has become increasingly widespread in recent years. The similarities with brassica crops add to the difficulty to control the weed as well as plant characteristics and growth habits.
In vegetables, Kerb can control shepardspurse when the product placed in the seed germination area close to the surface. If the product leaches the efficacy decreases. Dacthal, Prefar, Prowl, and Balan are less effective on this weed1.
Shepherspurse has proliferated in alfalfa recently and we are conducting some trials for its control. The results will be shared with PCAs and posted in our website when we finish collecting the data.


Fig 1. Images of an untreated plot compared to Raptor + Pursuit + Butyrac 200 +
NIS in alfalfa 4DAT.
Reference:
1.https://ag.arizona.edu/crops/vegetables/advisories/more/weed31.html
2.https://ipm.ucanr.edu/PMG/WEEDS/shepherdspurse.html#:~:text=Mature%20plant&text=Leaves%20vary%20in%20shape%20and,reduced%20in%20size%2C%20and%20stalkless.
Results of pheromone and sticky trap catches can be viewed here.
Corn earworm: CEW moth counts remain at low levels in all areas, well below average for this time of year.
Beet armyworm: Trap increased areawide; above average compared to previous years.
Cabbage looper: Cabbage looper counts decreased in all areas; below average for this time of season.
Diamondback moth: DBM moth counts decreased in most areas. About average for this time of the year.
Whitefly: Adult movement beginning at low levels, average for early spring.
Thrips: Thrips adult counts reached their peak for the season. Above average compared with previous years.
Aphids: Aphid movement decreased in all areas; below average for late-March.
Leafminers: Adults remain low in most locations, below average for March.