
When we go to the field to evaluate a crop, there are typically three fundamental points we need to take into consideration that include: 1) stage of growth, 2) general crop vigor and yield potential, and 3) anticipate the next stage of development and what we need to do in terms of field crop management. These considerations are commonly focused on aboveground crop evaluations. However, the root system is also an extremely important part of the crop condition to evaluate.
Root systems were described in a basic manner in a recent article on 2 May 2023 (UA Vegetable IPM Newsletter Volume 14, No. 9).
The effective root zone depth is the depth of soil used by the bulk of the plant root system to explore a soil volume and obtain plant-available moisture and plant nutrients. Effective root depth is not the same as the maximum root zone depth. As a rule of thumb, we commonly consider about 70% of the moisture and nutrient uptake by plant roots takes place in the top 24 inches of the root zone; about 20% from the third quarter; and about 10% from the soil in the deepest quarter of the root zone (Figure 1).
The small and very fine root hairs are the most physiologically active portion of a developing root system. It is important that the plants continue to develop and generate fresh young roots and an abundance of fine root hairs to maintain water and nutrient uptake.
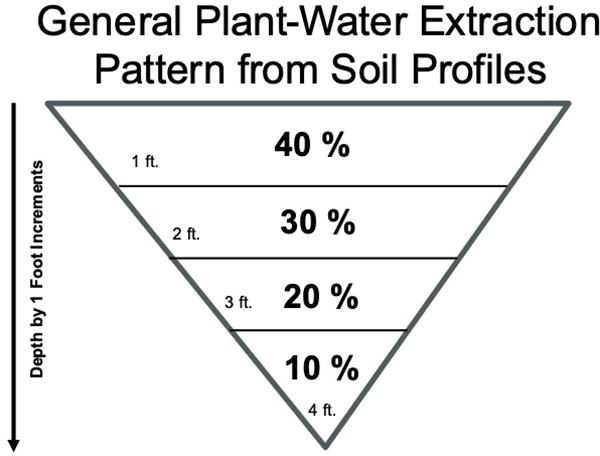
Figure 1. General pattern for plant-water and nutrient uptake from the soil profile.
It is important to point out that these general root development patterns are dependent on the nature of the soil profile in the fields. Soil profiles with compaction layers, as well as rock or caliche layers will limit root development and full exploration of the soil volume that the plants are capable of (Figure 2).
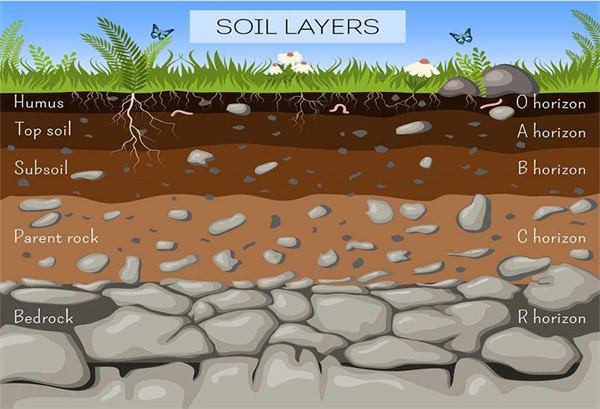
Figure 2. Generalized soil profile with major horizons.
Therefore, in scouting fields and making crop evaluations, examining the root systems is an important part of the process. Leafy green vegetable crops need to develop a marketable plant in a relatively short amount of time and a strong root system is essential.
It does take more time and effort to check root systems and it is a plant destructive process since we need to literally excavate the roots. So, it is also important to be careful of where and how we sample plants and the root systems in a field.
Crop species can vary significantly in their patterns of root development and it is important to know what is “normal” when evaluating crops in the field. An excellent reference for vegetable crop root system development is a 1927 publication by Dr. John E. Weaver and William E. Bruner from the University of Nebraska (Root Development of Vegetable Crops). This publication can be found at the following link:
https://soilandhealth.org/wp-content/uploads/01aglibrary/010137veg.roots/010137toc.html
A few basic examples from the Weaver and Bruner publication are provided in the following figures (Figures 3-10).
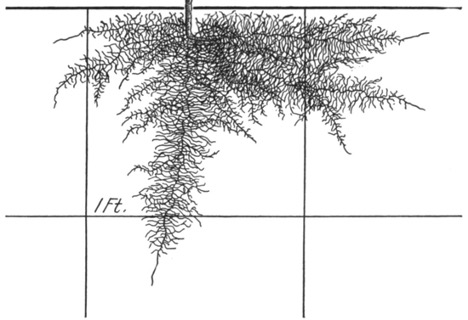
Figure 3. Cauliflower, 3 weeks after transplanting.
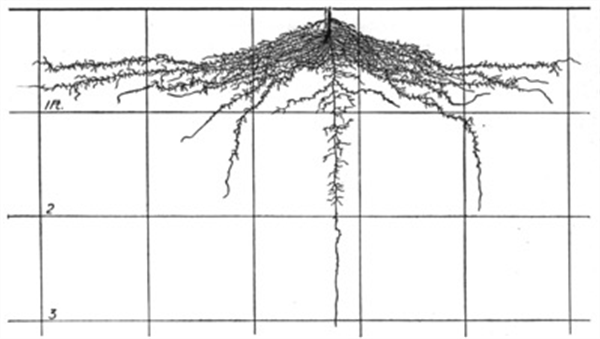
Figure 4. Cabbage roots, 55 days after transplanting.
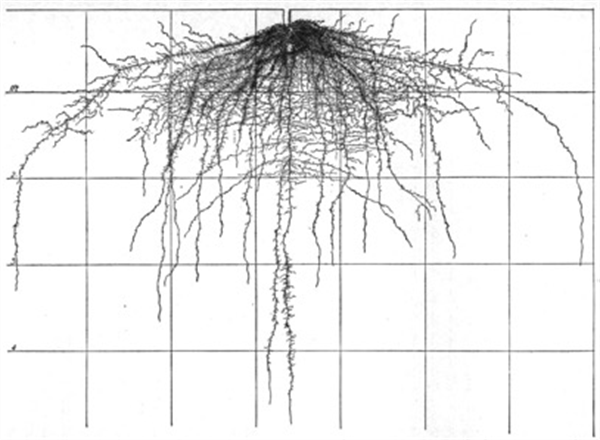
Figure 5. Cabbage roots, 75 days after transplanting.
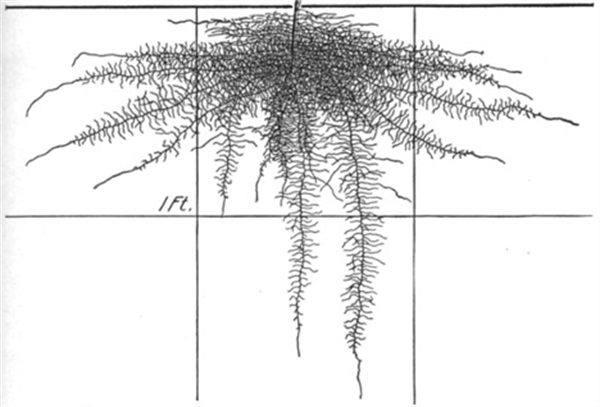
Figure 6. Pepper roots, 24 days.
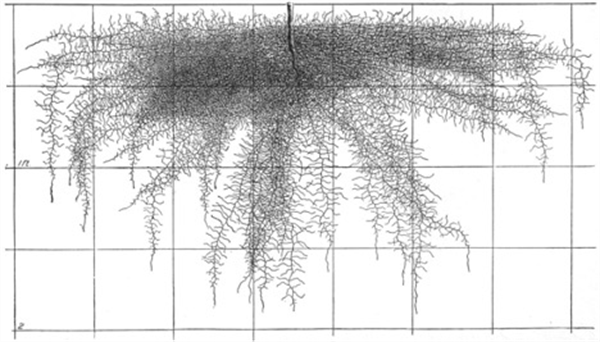
Figure 7. Pepper roots, 45 days (6 weeks).
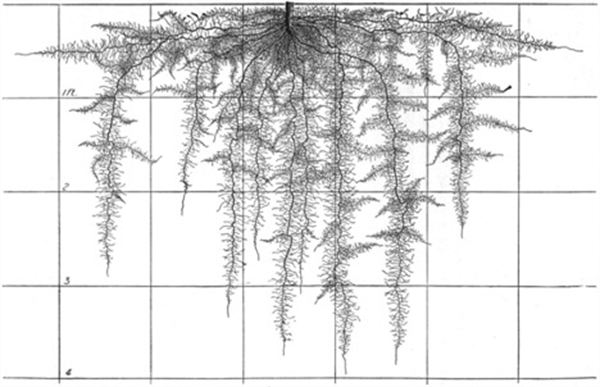
Figure 8. Pepper roots, mature.
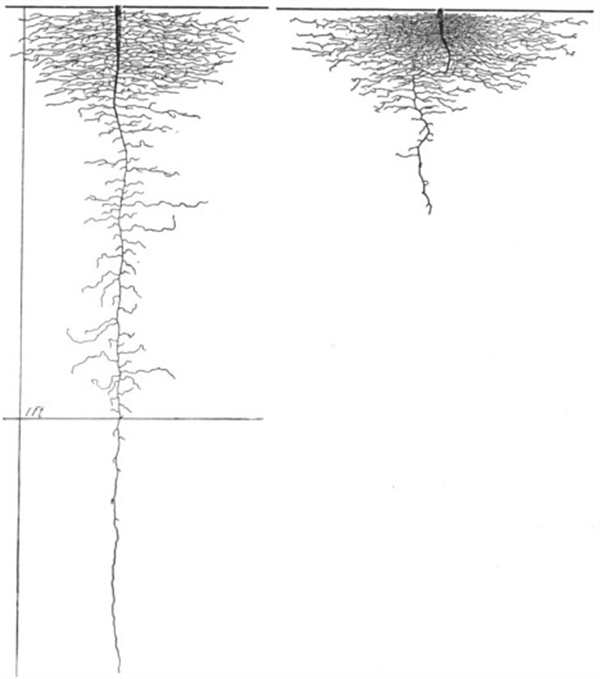
Figure 9. Lettuce roots, 3 weeks. The roots on the right were grown in compact soil, the roots on the
left were grown in loose/open soil.
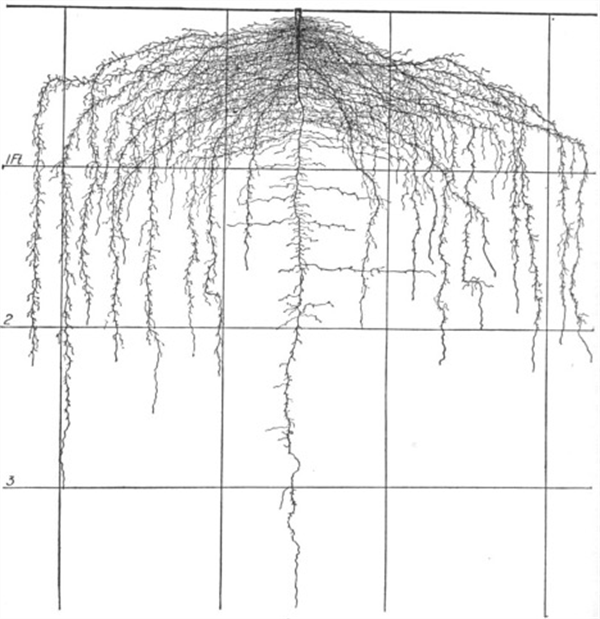
Figure 10. Lettuce roots, 60 days.
I am seeking samples of downy mildew on lettuce from around Yuma County to support the Michelmore Lab and their ongoing efforts to help characterize the downy mildew populations of the United States. The Michelmore Lab has led the charge on a survey of Bremia variants since 1980 and has been instrumental in demystifying the gene-for-gene nature of lettuce resistance to downy mildew.
Their group invites growers across the United States to submit downy mildew infected plant samples, which are then used to culture the Bremia on live host plants. The team then inoculates a panel of lettuce varieties carrying known resistance genes to determine the race of each isolate they receive. Identifying which races occur in which specific fields is essential to guiding the breeding of new resistant cultivars and maximizing the effectiveness of host-based genetic disease management. The data obtained from these tests are also used to designate new Bremia races through the International Bremia Evaluation Board.
Your contribution will help breed better lettuce for Yuma. This means less breakdown of resistance in the field, and better yields for Yuma growers. To facilitate these submissions the Yuma Plant Health Clinic will be setting up a separate drop-off point and submission sheet for downy mildew sample submissions in the same hallway we use for standard plant diagnostic submissions. The drop-off point will be clearly labelled and consist of a chest-style refrigerator and printed copies of the submission form. It is vital to keep these samples cool so they remain viable for future inoculations, so please place your samples inside of the refrigerator before you leave.
Shipping will be handled by the clinic. All we ask is that you fill out the submission form as completely as you can. An example of the questions that are asked in that form so you can prepare ahead of time can be found HERE .Fig. 1. Finger weeders removing a large, in-row Palmer Amaranth plant in cotton – slow motion video. (Video credit: Kyle R. Russel, Texas A&M University. Cultivator design and setup credit: Carl Pepper, Lubbock, TX). Click here or on the image to see the video.
On Thursday February 23, 2023, the afternoon the SWAS breakout sessions dedicated to Integrated Weed Management and Impact to Desert Agriculture (Located at AS 113) will have participation from Jesse Richardson from Corteva Agriscience. He will give us the historic perspective of Kerb Chemigation research. Samuel Discua Duarte from the U of A has conducted a weed survey for INSV virus hosts for a couple of years and will inform the results in his lecture.
Jose L. Carvalho de Souza from the University of Arizona Maricopa Agricultural Center will give us a lecture on the management of Palmer amaranth, the King of weeds. And I will inform some of the results from our Prefar trials at the Yuma Agricultural Center.
You are cordially invited to the session as well as the other breakout Southwest Ag Summit sessions organized by our Arizona Vegetable IPM Team…hope to see you there!.
INTEGRATED WEED MANAGEMENT AND IMPACT TO DESERT AGRICULTURE
(Requesting 2 CEU and 2 CCA)
Location: AS 113
Sponsor: Green Valley Farm Supply, Inc.
1:30-2:00p Insights from Early Kerb Chemigation Research
Jesse Richardson, Corteva Agriscience, Mesa, AZ
2:00-2:30p Survey of Weeds as Hosts of INSV in Yuma County – Results From Two Year Survey
Samuel Discua Duarte, University of Arizona, Yuma,AZ
2:30-3:00p Integrated Weed Management – Palmer Amaranth
Jose L. Carvalho de Souza, University of Arizona, Maricopa, AZ
3:00-3:30p Herbicide Evaluations at Yuma Agricultural Center
Marco Pena, University of Arizona, Yuma, AZ
Moderated by Marco Pena