
All plants have distinct habits of growth and development as a function of time (phenology). Plant growth is a direct response to environmental conditions, particularly temperature and in some cases daylength. As a result, tracking plant growth and development as a function if temperature conditions is a much more reliable method than the calendar.
In a recent edition of this newsletter on 4 April 2023, I presented a cantaloupe phenological (crop growth and development) model based on heat units accumulated after planting (HUAP, 86/55 Fo thresholds) as shown in Figure 1.
Phenological models can serve as good tools for improving crop management (e.g., fertilization, irrigation, harvest scheduling, pest management activities, and labor, etc.) by identifying and predicting important stages of crop growth and development and harvest dates.
Included in our work with the development of this phenological model, we have also conducted nutrient uptake and water use studies to develop a better understanding of nutrient and water demand for desert cantaloupe production (Silvertooth, 2003; Soto et al., 2006; and Soto, 2012).
Figure 2 presents the nitrogen (N) uptake and portioning patterns for desert cantaloupes (melons), (Silvertooth, 2003 and Soto et al. 2006). The total N uptake for cantaloupes was found to be approximately 140 lbs. N/acre. The maximum N flux (N uptake/day) period extends from early fruit development (crown stage) to the netting stage.
Water use by desert cantaloupe production was also measured in these studies and patterns of water use followed the crop coefficient (Kc) patterns provided by the Arizona Meteorological Network (AZMET) and conformed to the Kc values from FAO 56 (Allen et al., 1998 and Grattan et al., 1998).
Considering N uptake and water demand patterns in relation to cantaloupe crop phenology, we can insert the overlaps as shown in Figure 1, with the red and blue lines for N and water management, respectively. Maximum N demand occurs from approximately 500 to 1,000 HUAP, which coincides with primary fruit development.
The N application window is recommended in advance of the optimum N uptake period to provide for N mineralization and the plant-available forms of N for plant uptake and utilization.
The N application window for optimum N uptake is from approximately 300 to 800 HUAP, which is from early flowering (crown blooms) to the netting stage of the crown fruit.
Early and split applications during the N application window of cantaloupe crop development can help achieve optimum utilization and higher efficiencies of fertilizer N inputs.
The period of maximum water demand extends from early fruiting stages of development through the maturation of the crown fruit, 300 to 1300 HUAP.
Considering the current water shortages and high prices of fertilizers, we have sufficient motivation to manage our crop production systems with the highest efficiency possible. Understanding crop water and nutrient demand for each crop we are working with and using that knowledge to manage our crops most effectively, is to our benefit agronomically, economically, and environmentally.
Nitrogen is the plant nutrient required in largest amounts by crops and it is important for us to manage N inputs for a crop in a careful and deliberate manner. Water and N interactions are a critical aspect of crop growth, development, and management in any system, but particularly in an irrigated crop production system.
I encourage those who are working with spring cantaloupe production this season to test and evaluate this crop phenology model, particularly in relation to nutrient and water management under field conditions with various planting dates, varieties, and soil types. We appreciate your feedback.
Note: Up to date weather data, including HU accumulations since 1 January can be found for all of the Arizona Meteorological Network (AZMET) locations at the following website: https://ag.arizona.edu/azmet/
References:
Grattan, S.R., W. Bowers, A. Dong, R.L. Snyder, J.J. Carroll, and W. George. 1998. New crop coefficients estimate water use of vegetables, row crops. California Agriculture 52(1):16-21. https://doi.org/10.3733/ca.v052n01p16
Allen, R.G., L.S. Pereira, D. Raes, and M. Smith. 1998. Crop evapotranspiration - Guidelines for computing crop water requirements - FAO Irrigation and drainage paper 56. Food and Agriculture Organization of the United Nations. Rome (FAO). https://www.fao.org/3/x0490e/x0490e0b.htm
Silvertooth, J.C. 2003. Nutrient uptake in irrigated cantaloupes. Annual meeting, ASA-CSSA-SSSA, Denver, CO.
Soto, R. O. 2012. Crop phenology and dry matter accumulation and portioning for irrigated spring cantaloupes in the desert Southwest. Ph.D. Dissertation, Department of Soil, Water and Environmental Science, University of Arizona.
Soto-Ortiz, R., J.C. Silvertooth, and A. Galadima. 2006. Nutrient uptake patterns in irrigated melons (Cucumis melo L.). Annual Meetings, ASA-CSSA-SSSA, Indianpolis, IN.

Figure 1. Heat Units Accumulated After Planting (HUAP,86/55 °F)
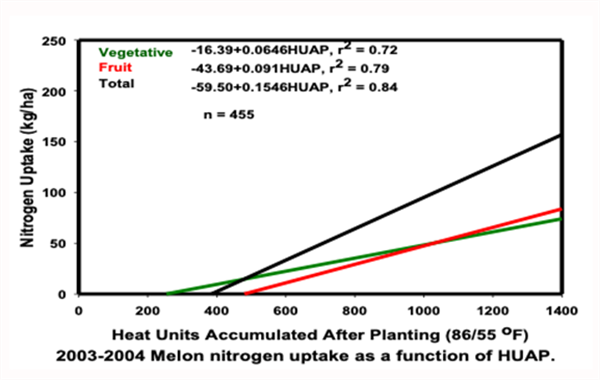
Figure 2. Cantaloupe (melon) N uptake and partitioning patterns. (Soto, Silvertooth,
and Galadima 2006). Note: kg/ha * 0.89 = lbs/acre
I hope you are frolicking in the fields of wildflowers picking the prettiest bugs.
I was scheduled to interview for plant pathologist position at Yuma on October 18, 2019. Few weeks before that date, I emailed Dr. Palumbo asking about the agriculture system in Yuma and what will be expected of me. He sent me every information that one can think of, which at the time I thought oh how nice!
When I started the position here and saw how much he does and how much busy he stays, I was eternally grateful of the time he took to provide me all the information, especially to someone he did not know at all.
Fast forward to first month at my job someone told me that the community wants me to be the Palumbo of Plant Pathology and I remember thinking what a big thing to ask..
He was my next-door mentor, and I would stop by with questions all the time especially after passing of my predecessor Dr. Matheron. Dr. Palumbo was always there to answer any question, gave me that little boost I needed, a little courage to write that email I needed to write, a rigid answer to stand my ground if needed. And not to mention the plant diagnosis. When the submitted samples did not look like a pathogen, taking samples to his office where he would look for insects with his little handheld lenses was one of my favorite times.
I also got to work with him in couple of projects, and he would tell me “call me John”. Uhh no, that was never going to happen.. until my last interaction with him, I would fluster when I talked to him, I would get nervous to have one of my idols listening to ME? Most times, I would forget what I was going to ask but at the same time be incredibly flabbergasted by the fact that I get to work next to this legend of a man, and get his opinions about pest management. Though I really did not like giving talks after him, as honestly, I would have nothing to offer after he has talked. Every time he waved at me in a meeting, I would blush and keep smiling for minutes, and I always knew I will forever be a fangirl..
Until we meet again.
Over the last couple of years, we identified and developed two high speed, precision spot sprayers to apply herbicides to weeds with minimal off-target spray while traveling at speeds that are viable for commercial vegetable farming operations (2 mph). The first is a spray assembly designed for controlling in-row weeds that are close to crop plants (1-cm spot spray resolution). The second is a high precision (sub-centimeter spot spray resolution) sprayer designed for spot spraying cotyledon stage weeds in spinach and leaf lettuce crops. We tested the device in the laboratory and found that weed control efficacy was greater than 95% (3 species), percentage of off-target spray was less than 3% and no crop injury was observed. The work was presented at the 2021 California Weed Science Society Annual Meeting. Click here or on the image below ) to view the presentation and see video of the devices in action.
We are now working towards developing a high precision, automated/robotic weeding machine for vegetable crops. This fall, Evan McGinnis, Biosystems Engineering Ph.D. candidate, initiated work to develop AI-based imaging software for detecting and targeting weeds. Once developed, the software will be integrated with the precision spot sprayer and tested in the field. Stay tuned for updates!
Acknowledgements
This work is supported by the Arizona Specialty Crop Block Grant Program and the USDA NIFA Specialty Crops Research Initiative USDA-NIFA-SCRI-004530. We greatly appreciate their support. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture.
Fig. 1. Images from “Centimeter Scale Resolution Sprayer for Precision In-Row Weed Control” presentation given at the 2021 California Weed Science Society Annual Meeting. Click here or on image to view the presentation.
As we continue to be impacted by the drought in Arizona with a 21% reduction in the Colorado River water allocation, we need to reconsider every option for water conservation in our agricultural operations.
We know that weeds compete with our crops for water, nutrients, and space causing yield reductions. However, how much water are we loosing due to high weed infestations?
Some researchers have concluded that weeds use more water than various crops and consider them “water wasters”. Therefore, good weed control can contribute to raise available water for our crops. Transpiration of some of the most common annual weeds is approximately four times higher than crop plants. It has also been reported that weeds use up to three times the amount of water to produce a pound of dry matter. A study showed “common lambsquarters (Chenopodium album) requires 658 pounds of water to produce one pound of dry matter, common sunflower (Elianthus annus) requires 623 pounds, and common ragweed 912 pounds, compared with 349 pounds for corn and 557 pounds for wheat1.” It has been reported that increase from 0 - 8 plants / row meter of Palmer amaranth (Amaranthus palmeri) densities in corn decreased soil water available and the water use efficiency (WUE) of corn.
Uncontrolled weed growth can add direct irrigation costs of more than $50/ha while even weed densities below economic thresholds can add ~$20/ ha in production costs depending upon the cropping system and water cost (Norris,1996).
Under stress condition such as we experience yields can be reduced more 50% just by moisture competition. Other factors that influence water loss are weed densities, transpiration rate, other weed characteristics like root system and depth. For example, perennial weeds with a well-established root system are more drought resistant because they can explore better the soil profile.
Some report that weeds can potentially cause 34 percent of crop loss worldwide. We have seen how weeds cut the water flow in irrigation ditches and cause more evaporative loss. We believe weed control is essential for water conservation purposes and further research is needed in this matter.
Reference:
1. https://www.researchgate.net/profile/Hussein-Abouziena-2/publication/278020092_Water_loss_by_weeds_A_review/links/5578c26608ae752158703bdc/Water-loss-by-weeds-A-review.pdf