With the planting of every crop there is a time of anticipation as we wait and watch for germination, seedling emergence, and the development of a good stand of plants to start the crop season. Successful seedling establishment is the first critical step for the production of any crop and it determines the foundation for either the success or failure of the future harvest. So, it is worthy to review some fundamentals associated with the process of seed germination and seedling establishment.
Most crops produced worldwide begin with the planting of a seed and management to sufficiently establish a satisfactory population of new plants in the field. Good seed germination and establishment is an essential and critical first step in the production of a good crop. Most crop seeds are desiccation tolerant while dormant and as a result can be stored and transported while preserving their ability to grow. Seeds carry the full genetic complement of the crop and serve as the genetic delivery mechanism for a crop production system. Farmers require seeds that will provide for a reliable and successful establishment of a crop.
An interesting aspect of seed germination and establishment is that seeds generally represent the condition of highest degree of resistance to extreme environmental conditions, while seedlings are most sucseptible. Viable seeds are dormant living organisms that contain living, embryonic tissue that is essential for germination. All fully developed seeds contain an embryo and a reserve supply of carbohydrates and nutrients to carryout germination and establishment and the full “package” is contained within a seed coat.
Seeds are stimulated to “wake up” and start the germination process when soil moisture and temperature conditions are appropriate for them to grow. The three cardinal points of vital activity for germination are: 1) a minimum temperature, below which no activity occurs, 2) an optimum temperature at which the highest germination occurs, and 3) a maximum temperature above which no germination takes place.
All seeds need correct moisture and temperature environments (Table 1) to initiate internal biochemical processes associated with the initation of germination. Soil-water content generally needs to be at least approximately 50–75% of field capacity. A fine-textured seedbed and good seed-to-soil contact are very imporant for optimal germination.
Good soil aeration that facilites good gas exchange between the germinating embryo and the soil environment is very important. Seeds respire just like any other living organism. Germinating seeds need oxygen and they produce carbon dioxide (CO2). The CO2 needs to be able to diffuse away from the seed and if the soil is compacted or saturated, aeration is inhibited and the CO2 respiration will not be able to dissipate to the atmosphere, and as a result the seeds can suffocate in the soil.
There are three major steps in the initial process of seed germination. These include: 1) imbition, 2) interim or lag phase, and 3) radicle and root emergence.
In the imbibition process seeds rapidly absorb water through the hilum of the seed and the seed coat will then swell and soften.
In the interim or lag phase the seeds internal biochemical and physiological system goes into action. Cells begin respire with this physiological activity and the seed begins to metaboloize its storehouse of energy and nutrient reserves to manufacture proteins other essential metabolites.
The radicle is the very first element of the rooting system that is developed and it is the first thing to emerge from the seed. In the field, we often refer to the radicle as the first “stinger” root that is developed. In the field, it is good to dig into the seedbed and examine early seedling development and look for healthy radicles, with clean, white, and turgid tissue.
In the process of radicle and root emergence, the cells of these tissues start to elongate and divide, the seed continues to imbibe water, and then it begins to extend out and away from the seed into the soil. These early root tissues, specifically the fresh root hairs become quickly capable of water and nutrient uptake. The young root tissues are very sensitive to the soil environment, particularly the soil solution. It is in this stage for example when seedlings are often very sensitive to salinity in the soil solution and disease infections.
When the radicle begins absorbing water from the soil solution, the seedling gains the capacity to extend a shoot from the seed towards the soil surface for emergence. In dicotyledonous (dicot) plants (generally referred to as broadleaf plants) the two seed leaves are extended above the soil for emergence. The section below the shoot and the cotyledons is referred to as the “hypocotyl” and the section of the shoot above the cotyledons is the “epicotyl”, Figure 1.
There are two basic methods of emergence for plants. In most dicot plants, a hook is formed in the section of shoot below the cotyledons which serves to pull the cotyledons and the growing point of the new plant up through the soil. Upon the reaching the soil surface, the hook will straighten and pull the cotyledons (seed leaves) and shoot tip into the air. For the farmer or agronomist in the field, that is a good sight to see. This method of emergence is called “epigeous or epigeal germination”. Examples of epigeal germination are cotton, sunflower, castor, and bean (common bean), Figure 1.
In other plants a process of “hypogeous or hypogeal germination” takes place. In hypogeous germination, the hypocotyl remains short and the cotyledons do not emerge from the seed and remain underground. Instead, the radicle and epicotyl axis will elongate and extend out of the seed coat. The epicotyl grows up through the soil and emerges above the soil surface and forms a epicotyl or “plumule” for the first leaf, Figure 2a and b.
All monocotyledons plants such as maize, rice, wheat, and coconut show hypogeal germination. It is also interesting to note that some dicot plants show hypogeal germination such as groundnut, gram, and peas.
In general, plants with epigeal germination, most dicot or broadleaf plants, are more sensitive to soil crusting and environmental inhibitions to emergence. So it is good to know what type of germination and emergence characteristics are natural for a given crop species and to track seedling development in the field accordingly.
As we begin any new crop season in the field and plant the seeds, it is good to review the basic process of seed germination and establishment. We can easily take all of this for granted until something goes wrong and we have problems in the field with emergence. After planting, it is good to monitor in-field seedling conditions and progression of growth and development, both above and below ground, and provide good management that supports the young plants as they get established and start their growing cycle.
Table 1. Soil temperature (oF) for crop germination. Adapted from Kemble and Musgrove (2006).
|
Crop |
Minimum oF |
Optimal Range oF |
Optimum oF |
Maximum oF |
|
Beet |
40 |
50–85 |
85 |
85 |
|
Cabbage |
40 |
45–95 |
85 |
100 |
|
Cauliflower |
40 |
45–85 |
80 |
100 |
|
Celery |
40 |
60–70 |
70 |
85 |
|
Chard |
40 |
50–85 |
85 |
95 |
|
Cucumber |
60 |
60–95 |
95 |
105 |
|
Eggplant |
60 |
75–90 |
85 |
95 |
|
Lettuce |
35 |
40–80 |
75 |
85 |
|
Melons |
60 |
75–95 |
90 |
100 |
|
Onion |
35 |
50–95 |
75 |
95 |
|
Parsley |
40 |
50–85 |
75 |
90 |
|
Pepper |
60 |
65–95 |
85 |
95 |
|
Pumpkin |
60 |
70–90 |
90 |
100 |
|
Spinach |
35 |
45–75 |
70 |
85 |
|
Squash |
60 |
70–95 |
95 |
100 |
|
Tomato |
50 |
70–95 |
85 |
95 |

Figure 1. Seed germination and plant establishment for a plant with epigeal
germination and emergence, which is common with most dicot or broadleaf
plants.
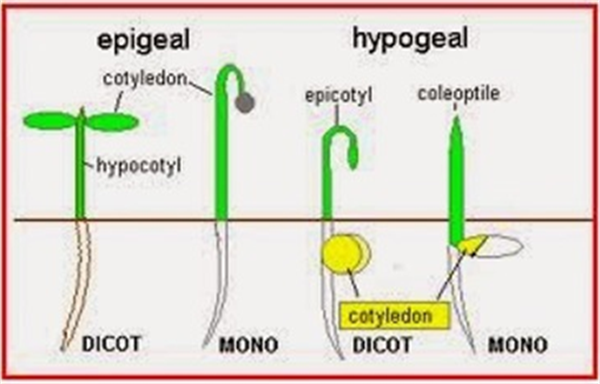
Figure 2a. Examples and comparison of epigeal and hypogeal germination.
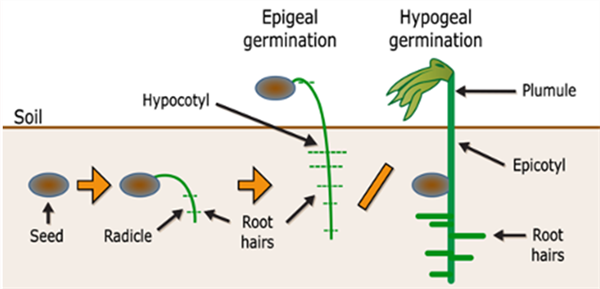
Figure 2b. Examples and comparison of epigeal and hypogeal germination.
I hope you are frolicking in the fields of wildflowers picking the prettiest bugs.
I was scheduled to interview for plant pathologist position at Yuma on October 18, 2019. Few weeks before that date, I emailed Dr. Palumbo asking about the agriculture system in Yuma and what will be expected of me. He sent me every information that one can think of, which at the time I thought oh how nice!
When I started the position here and saw how much he does and how much busy he stays, I was eternally grateful of the time he took to provide me all the information, especially to someone he did not know at all.
Fast forward to first month at my job someone told me that the community wants me to be the Palumbo of Plant Pathology and I remember thinking what a big thing to ask..
He was my next-door mentor, and I would stop by with questions all the time especially after passing of my predecessor Dr. Matheron. Dr. Palumbo was always there to answer any question, gave me that little boost I needed, a little courage to write that email I needed to write, a rigid answer to stand my ground if needed. And not to mention the plant diagnosis. When the submitted samples did not look like a pathogen, taking samples to his office where he would look for insects with his little handheld lenses was one of my favorite times.
I also got to work with him in couple of projects, and he would tell me “call me John”. Uhh no, that was never going to happen.. until my last interaction with him, I would fluster when I talked to him, I would get nervous to have one of my idols listening to ME? Most times, I would forget what I was going to ask but at the same time be incredibly flabbergasted by the fact that I get to work next to this legend of a man, and get his opinions about pest management. Though I really did not like giving talks after him, as honestly, I would have nothing to offer after he has talked. Every time he waved at me in a meeting, I would blush and keep smiling for minutes, and I always knew I will forever be a fangirl..
Until we meet again.
Interested in the latest ag technologies? FIRA USA and a number of other entities are organizing a 3-day event October 18-20 in Freson, CA focused on autonomous ag robotics and technologies. The event includes keynote speakers, break out sessions, a trade show and in-field demos of harvesting, weeding, thinning, and planting robots. The emphasis is on specialty crops so it should be very interesting program. For more information, click here.

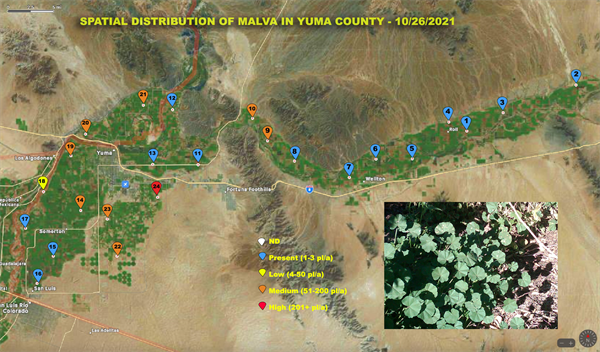
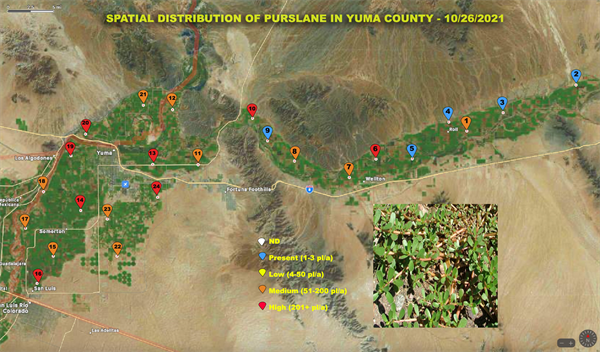
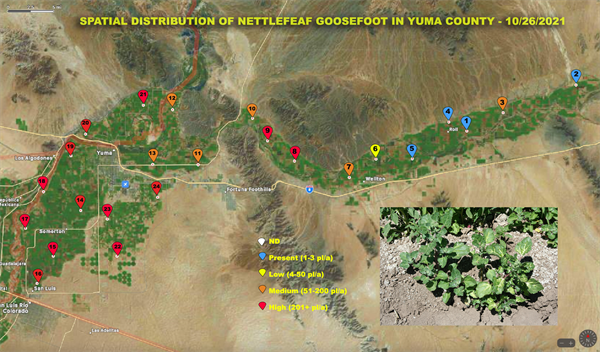
Bi-Weekly Weed Survey of Yuma County starts today
A Bi-Weekly survey of weeds of agricultural areas in Yuma County began two weeks ago and is reported in today’s edition of the IPM Updates. Survey results will continue to be reported in Vegetable IPM Updates every two weeks. This survey was initiated and will be conducted by Marco Pena.
The purpose of the survey is to provide information to help you make weed management decisions and will include weed species, location and infestation level. Twenty four points have been marked for sampling. They are distributed from San Luis to Texas Hill. A three to five acre radius will be surveyed around each point. Results will be reported on an aerial photograph of the county. A separate map will be provided for each species found. We know that we can’t find every weed out there across the county and will make an interactive map so that you can include infestations that we have missed. Just send us an image of the weed species and the GPS location from your phone and we will add it to the map.
We hope that this will be ongoing project that will alert you to what weeds we have found and create an historical perspective for changes over time. We will be adding more species as the project continues and we appreciate your input on how this program can be modified and improved so please let us know what you think.
The scale used on this survey for the infestation level on each weed is color coded as follows:
|
Color Code |
Infestation Level |
Plants / acre |
|
White |
ND: Not Detected |
0 |
|
Blue |
P: Present |
1-3 pl/ac |
|
Yellow |
L: Low |
4-50 pl/ac |
|
Orange |
M: Medium |
51-200 pl/a |
|
Red |
H: High |
Above 200 pl/ac |
The following image contain the location of our 24 sampling points. To access all the Yuma county weed maps with other species including some that weeds found to host the INSV virus please visit Spatial Distribution of weeds in Yuma County.