
Cantaloupes (Cucumis melo L.) or “melons” are one of the important spring and fall vegetable crops of Arizona and the desert Southwest. Technically, “true” cantaloupes are rough, warty fruit, primarily grown in Europe. On a production scale, cantaloupes are not grown commercially in the United States. However, in the United States “cantaloupe” has become a general name of all netted, musk-scented melons (Simmone et al., 1998 and Soto, 2012).
Cantaloupes in the U.S. are divided into eastern and western types. The eastern type is characterized by round-shaped fruits, usually about five to seven pounds, sutured (sutures are the green lines that divide the rind into several sections), with variable levels of netting (netting is the network of cork-like marks that cover the rind), and large seed cavities. The western type is characterized by oval-shaped fruits of three to four pounds, sutureless, and a coarsely netted rind (Simmone et al., 1998 and Soto, 2012).
According to the United States Department of Agriculture (USDA) National Agricultural Statistical Services (NASS), the harvested Arizona cantaloupe acreage from 1992 to 2021 has ranged from 13,200 to 23,300 acres with an estimated production value ranging from $38 million to $82.5M. There were 19,300 acres of Arizona cantaloupes in 2021 (USDA, 2021). Most of the Arizona cantaloupe production takes place in Yuma, Maricopa, and Pinal Counties. Among the nine states with recorded cantaloupe production, Arizona commonly ranks second to California in acres and total production. (USDA, 2021 and Murphree, 2015).
Being able to accurately describe and predict important stages of crop growth and development (crop phenology) and harvest dates is important for improving crop management (e.g. fertilization, irrigation, harvest scheduling, pest management activities, labor and machinery management, etc.). Since plants operate on “thermal time”, they have no regard for calendars or time as we commonly measure it. So, we find it is best to monitor and predict plant development based on the actual thermal conditions in the plant’s environment. Various forms of temperature measurements and units commonly referred to as heat units (HU), growing degree units (GDU), or growing degree days (GDD) have been utilized in numerous studies to predict phenological events for many crop plants (Baker and Reddy, 2001 and Soto, 2012).
Boswell (1929) first documented the concept of heat summations relative to vegetable crop production in 1929. Thereafter, HU accumulation techniques have been successfully applied to numerous vegetable production systems like cantaloupe (Baker et al., 2001).
The use of HU accumulations has been shown to be an efficient technique for modeling and predicting growth stages in crops (such as cantaloupes) as compared with the traditional days after planting (DAP) method, since variations among seasons and locations can be better normalized using heat units accumulated after planting (HUAP) calculations rather than DAP.
For more than 35 years we have had the benefit of excellent weather data collection in Arizona from the Arizona Meteorological Network (AZMET), which was first developed and directed by Dr. Paul Brown. For warm season crops, such as cantaloupes, we have been working HUs with both upper and lower temperature thresholds (86/55 oF), as first described by Baskerville and Emin (1969) and shown in Figure 1 (Brown, 1989). We have successfully developed crop phenology models using HUs with 86/55 oF thresholds for other common warm season crops in the desert Southwest, such as cotton (Silvertooth, 2001) and New Mexico type chiles (Silvertooth et al., 2010).
Baker et al., (2001) developed a muskmelon phenology model that could be run with easily obtainable weather station data and used by growers to quantify phenological development and aid in projecting harvest dates. The average model accuracy in predicting harvest dates ranged between 1 to 3 days for the data set used to construct the model. Also, after the evaluation of the performance of two GDD models to predict commercial harvest dates in 30 commercial melon fields in California, Hartz (2001) found that the two models were useful in predicting the date of harvest initiation. The standard deviation for the prediction of harvest date from emergence date represented between 2-3 days of normal growing-degree-day accumulation.
Beginning in 2000, we began working in Arizona to develop and test a phenology model for desert cantaloupe production. Following data collection from many spring cantaloupe fields, primarily in the Yuma area, we were able to develop and test the basic cantaloupe phenology model shown in Figure 2 (Silvertooth, 2003; Soto et al., 2006; and Soto, 2012).
I encourage those who are working with spring cantaloupe production this season to test and evaluate this crop phenology model in the field under various planting dates, varieties, and conditions. We appreciate your feedback.
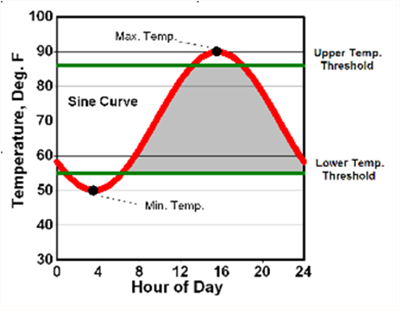
Figure 1. Graphical depiction of heat unit computation using the single sine
curve procedure. A sine curve is fit through the daily maximum and minimum
temperatures to recreate the daily temperature cycle. The upper and lower
temperature thresholds for growth and development are then superimposed
on the figure. Mathematical integration is then used to measure the area
bounded by the sine cure and the two temperature thresholds (grey area). (Brown, 1989)
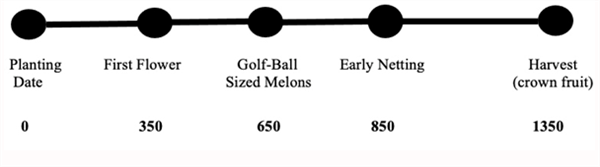
Figure 2. Heat Units Accumulated After Planting (HUAP, 86/55 oF)
References:
Baker, J.T., and V.R. Reddy. 2001. Temperature effects on phenological development and yield of muskmelon. Annals of Botany. 87:605-613.
Baskerville, G.L., and P. Emin. 1969. Rapid estimation of heat accumulation from maximum and minimum temperatures. Ecology 50:514-517.
Boswell, V. R. 1929. Factors influencing yield and quality of peas. Maryland Agric. Exp. Sta. Bull. 306.
Brown, P. W. 1989. Heat units. Ariz. Coop. Ext. Bull. 8915. Univ. of Arizona, Tucson, AZ.
Hartz, T.K. 2001. Development and testing of a growing degree day model to predict melon harvest time. California Melon Research Board. 2001. Annual Report.
Murphree, J. 2015. Fun Statistics about Arizona Agriculture’s Melons and Sweet Corn. Arizona Farm Bureau https://www.azfb.org/Article/FunStatistics-about-Arizona-Agricultures-Melons-and-Sweet-Corn
Silvertooth, J.C. 2001. Following cotton development over the fruiting cycle. University of Arizona Cooperative Extension Bulletin No. AZ 1206.
Silvertooth, J.C. 2003. Nutrient uptake in irrigated cantaloupes. Annual meeting, ASA-CSSA-SSSA, Denver, CO.
Silvertooth, J.C., P.W. Brown, and S. Walker. 2010. Crop Growth and Development for Irrigated Chile (Capsicum annuum). University of Arizona Cooperative Extension Bulletin No. AZ 1529
Simonne, A., E. Simonne, R. Boozer, and J. Pitts. 1998. A matter of taste: Consumer preferences studies identify favorite small melon varieties. Highlights of Agricultural Research. 45(2):7-9.
Soto, R. O. 2012. Crop phenology and dry matter accumulation and portioning for irrigated spring cantaloupes in the desert Southwest. Ph.D. Dissertation, Department of Soil, Water and Environmental Science, University of Arizona.
Soto-Ortiz, R., J.C. Silvertooth, and A. Galadima. 2006. Nutrient uptake patterns in irrigated melons (Cucumis melo L.). Annual Meetings, ASA-CSSA-SSSA, Indianpolis, IN.
USDA Stats: 2021 State of Agriculture Overview.
https://www.nass.usda.gov/Quick_Stats/Ag_Overview/stateOverview.php?state=ARIZONA
Hi, I’m Chris, and I’m thrilled to be stepping into the role of extension associate for plant pathology through The University of Arizona Cooperative Extension in Yuma County. I recently earned my Ph.D. in plant pathology from Purdue University in Indiana where my research focused on soybean seedling disease caused by Fusarium and Pythium. There, I discovered and characterized some of the first genetic resources available for improving innate host resistance and genetic control to two major pathogens causing this disease in soybean across the Midwest.
I was originally born and raised in Phoenix, so coming back to Arizona and getting the chance to apply my education while helping the community I was shaped by is a dream come true. I have a passion for plant disease research, especially when it comes to exploring how plant-pathogen interactions and genetics can be used to develop practical, empirically based disease control strategies. Let’s face it, fungicide resistance continues to emerge, yesterday’s resistant varieties grow more vulnerable every season, and the battle against plant pathogens in our fields is ongoing. But I firmly believe that when the enemy evolves, so can we.
To that end I am proud to be establishing my research program in Yuma where I will remain dedicated to improving the agricultural community’s disease management options and tackling crop health challenges. I am based out of the Yuma Agricultural Center and will continue to run the plant health diagnostic clinic located there.
Please drop off or send disease samples for diagnosis to:
Yuma Plant Health Clinic
6425 W 8th Street
Yuma, AZ 85364
If you are shipping samples, please remember to include the USDA APHIS permit for moving plant samples.
You can contact me at:
Email: cdetranaltes@arizona.edu
Cell: 602-689-7328
Office: 928-782-5879
For those interested in autonomous ag field robots, Future Farming (Misset Publisher, BV, Doetinchem, Netherlands) put together an informative video that features 16 commercially available units. A wide range of robots are highlighted – tool carriers, planters that geo-reference seed placement, several weeding machines and even a rock picker. The short, 3 minute 20 second video is interesting and a quick way to get up to date on these technologies.
It will be interesting to see what the future holds and whether these types of machines will become adopted in Arizona vegetable production. When discussing the high cost of automated weeding and other “smart” machines with a grower, he stated that the tractor driver was the cheapest part of the system. It’s a good point and one that’s hard to argue with.
Click here or on the image to see Commercial Autonomous Ag Robot video.
Weeds that are outside of fields can still be harmful to crop production. This does not include ditch banks or fallow fields. Non-crop weed control includes weeds around buildings, roadways, equipment yards, fence lines etc. It is different than fallow ground or ditch banks weed control and includes places where crops are not grown. Weeds in non-crop areas can be a source of weed seeds that blow or are carried into places where crops are grown. They can also be a refuge for insects, diseases and rodents. The reason that these herbicides are registered for non-crop areas is that they are broad spectrum and persistent. Some are registered for use in fields but at much lower rates than for non-crop areas. For instance, Diuron can be used on alfalfa at 1 to 1.5 qts/ac. but is used for non-crop areas at 12 qts/ac.
|
Herbicide |
Weeds Controlled |
Activity |
Soil Residual |
Comments |
|
Bromacil |
Broadleaves and grasses |
Preemergence |
1 to 2 years |
The nuclear option Should be incorporated with water. Keep away from roots of desirable vegetation. |
|
Hexazinone |
Broadleaves and grasses |
Preemergence and Postemergence |
1 to 2 years |
Keep away from roots of desirable vegetation. Incorporate with water. |
|
Diuron |
Broadleaves and grasses |
Preemergence and Postemergence
|
2 years |
Incorporate with water but do not leach into roots of desirable vegetation |
|
Prometon |
Broadleaves and grasses |
Preemergence and Postemergence
|
1 to 2 years |
Must be incorporated with water |
|
Tebuthiuron |
Broadleaves and grass. Good on hardwood trees |
Preemergence and postemergence |
2 years |
Incorporate with water. Contact with even on feeder root of desirable vegetation can be lethal |
|
Imazapyr |
Broadleaves and grasses |
Preemergence and post emergence |
1 year |
Primarily a postemergence herbicide but is readily absorbed by rootsif incorporated with water |
|
sulfometuron |
Broadleaves and grasses |
Preemergence and post emergence |
1 year |
Must incorporate with water |
|
Aminopyralid |
Broadleaves |
Preemergence |
1 year |
broadleaves only. Shorter soil residual for weeds but crops can not be planted for 1-2 years |
This time of year, John would often highlight Lepidopteran pests in the field and remind us of the importance of rotating insecticide modes of action. With worm pressure present in local crops, it’s a good time to revisit resistance management practices and ensure we’re protecting the effectiveness of these tools for seasons to come. For detailed guidelines, see Insecticide Resistance Management for Beet Armyworm, Cabbage Looper, and Diamondback Moth in Desert Produce Crops .
VegIPM Update Vol. 16, Num. 20
Oct. 1, 2025
Results of pheromone and sticky trap catches below!!
Corn earworm: CEW moth counts declined across all traps from last collection; average for this time of year.
Beet armyworm: BAW moth increased over the last two weeks; below average for this early produce season.
Cabbage looper: Cabbage looper counts increased in the last two collections; below average for mid-late September.
Diamondback moth: a few DBM moths were caught in the traps; consistent with previous years.
Whitefly: Adult movement decreased in most locations over the last two weeks, about average for this time of year.
Thrips: Thrips adult activity increased over the last two collections, typical for late September.
Aphids: Aphid movement absent so far; anticipate activity to pick up when winds begin blowing from N-NW.
Leafminers: Adult activity increased over the last two weeks, about average for this time of year.







