Considering everything we do to grow a crop in Arizona, besides perhaps selecting the variety of the crop to be grown, irrigation is clearly the most important agronomic (crop and soil) aspect of crop management. The fact that Arizona consistently produces the highest yields and quality of crops found anywhere is a testimony to the fact that Arizona growers understand this quite well and they are extremely good at managing irrigation.
Part of what has made Arizona farmers so productive is the constant quest to improve on our irrigation methods, particularly in terms of improving irrigation efficiency. We know there are costs agronomically, economically, and environmentally associated with over- or under-irrigation and we constantly strive to alleviate both forms of irrigation water loss and improve irrigation efficiencies.
Irrigation is often considered as an engineering event and that it is true regarding the conveyance of water from a source and getting it to the field. However, the management of irrigation in the field, and particularly the management of both the timing and amount of irrigation, is completely an agronomic decision and action because the objective is to replenish the plant-available water in the soil for the benefit of the crop.
We have access to many methods and technologies to help improve irrigation management and efficiency (Cotton Inc. Sensor-based scheduling; Ratliff et al., 1983). Irrespective of the technologies or methods being used, all successful methods of irrigation management must deal with two basic issues: 1) tracking crop water use and 2) the replenishment of plant-available water in the soil with an irrigation event (Allen et al., 1988; Datta et al., 2017; Hanson et al., 2000).
For efficient management of irrigation water, there are several aspects of the total water in a soil that are important to recognize and the primary water fraction of interest is referred to as the “plant-available soil-water” (PAW). Figure 1 provides a graphic illustration of three important components of soil water: 1) total water in the soil (TW), 2) PAW, and 3) and unavailable soil-water to plants.
A very simple way to describe PAW in a soil can begin by considering the soil with a “sponge” analogy. If we put a sponge under a water faucet and fully saturate it, then let it hang in suspension, we will see free water draining from the sponge for a short period of time. We can consider a soil profile similarly after an irrigation where the soil will saturate and there will be drainage of the water that the soil cannot physically hold, this is referred to as drainage water, the leaching fraction, or the “gravitational water” (GW). The TW water is retained in the soil after the drainage water has moved out of the wetted zone. The TW is held in the soil due to the matrix forces of the soil particles that adhere to that water, like the forces holding water in the saturated sponge analogy. The TW remaining in the soil after the GW has drained away, contains the PAW and the non-available water that the soil holds by matrix forces stronger than what the plant extract (Figures 1 and 2).
There are physical definitions to describe several critical levels of soil-water content and the forces holding water in the soil. For example, field capacity (FC) is the point at which all the GW water has drained out of a soil. Soil physicists have defined FC as a function of potential energy at -33 kPa (kilopascals). Similarly, the permanent wilting point (PWP) has been defined as the point at which all PAW has been extracted from a soil. The PWP is defined somewhat arbitrarily at a potential energy level of -1500 kPa. The more negative the potential energy value, the lower the soil water content and the stronger the water is held in thin layers by the soil particles.
For practical field management of soil water, it is important to focus on the PAW for each soil and crop. It is important to understand that plants are not all created equal in terms of their capacity to extract water from a soil and some plants can extract more water from a given soil than other plants. For example, native desert plants (xerophytic plants) can extract much more water from a soil than most crop plants. Compared to native desert plants, our crop plants are much more sensitive. Therefore, the PAW illustrated in Figures 1, 2, and 3 are general descriptions but they illustrate an important soil-plant relationship. Each plant species has its own capacity to extract water from the soil and we need to know and understand this characteristic of each crop that we are managing in the field as well as the water holding capacity of the soil. For example, lettuce and most leafy-green vegetable crops are more sensitive to water stress than crops like cotton, wheat, melons, alfalfa, sorghum, etc. Thus, leafy green vegetable crops must be maintained at a higher level of PAW to avoid water stress in contrast to some other crop plants such as cotton, wheat, etc. The PWP for these crops will occur much before the soil-water content reaches the point of -1500 kPa.
Crop plants will draw upon the PAW fraction to sustain themselves physiologically and manage against water stress. Crop demands for water change as a function of stage of growth and environmental or weather conditions. Our fundamental irrigation management goal is to monitor the depletion of PAW in the soil and schedule an irrigation at the proper time and rate to replenish the PAW before the crop goes into water stress. That means we also need to identify the critical level of PAW for the crop in the field to determine when an irrigation is needed so we can avoid water stress on the crop.
The fact that different soil types, determined by soil texture, have different total and PAW holding capacities is well illustrated in Figure 1 and Table 1. Thus, it is important to know the soil type prevalent in any field to gauge the amount of PAW that a soil can hold. This will help determine to what extent the soil-water can be depleted and the amount of irrigation water needed to replenish the PAW.
There are many methods and technologies to measure soil-water and PAW to assist in irrigation management. However, the most fundamental method is to sample a soil and estimate “plant-available” water status by “feel”, by literally holding the soil in your hand and estimating the amount of plant-available water that is present.
A good way to calibrate the feel method is to check the soil in a field a few hours after an irrigation event when the GW has drained away and the soil is at FC. The next critical point to identify by the feel method is the soil-water content just before the plants begin to show any sign of water stress. The difference between those two points is the PAW that we are managing in the field. That critical point in the soil-water content that we need to recognize for managing irrigations for crop plants will still have a significant amount of PAW left in the soil but that is because the goal in crop management is to avoid plant water stress, which is signaled by the plant with wilting. Thus, that critical point for irrigating most crop plants is to do so just before the plant begins to experience water stress and show any sign of wilting.
To determine the moisture level in the soil we need to dig down with a shovel, soil probe, or with a soil auger to at least a depth of about 6-12 inches to get a representative soil sample. In general, for many crops and certainly vegetable crops, if the soil forms a tight ball and leaves a wet outline on your hand when you squeeze it, we can delay irrigation until the ball of soil, while still slightly moist and cool to the touch, is dry enough that it begins to crumble at the edges. The depth and time of irrigation should be long enough to fill the entire root zone of the plant, which will be dependent on both the soil texture (Table 1) and the level of soil water depletion.
Tracking and managing PAW water in the soil is a fundamental acquired skill that serves as a check on the irrigation scheduling methods or technologies being used and it remains an extremely valuable skill for anyone managing irrigations. We have many technologies available for irrigation management but a farmer or agronomist’s ability to recognize and understand these relationships and develop the necessary field skills is fundamental to good crop management. Identifying critical points in soil water content and plant growth, such as FC, PAW, and PWP by sampling a soil and making good estimates in the field by watching the crop and noting the various stages in crop development, serve a farmer or agronomist as a good standard and check on any technology being employed for irrigation management.
Farmers and agronomists learn to watch a crop in the field and recognize very subtle changes in leaf color and condition and they can also recognize the crop response and relationship to soil water content. That gives them the capacity to anticipate the optimum timing for the next irrigation. Farmers and agronomists understand these relationships among crop plants, soil water content, and the critical points for irrigation management. Irrigation management is an example of the blending of art and science that goes into good crop management, and it is certainly important in Arizona agriculture.
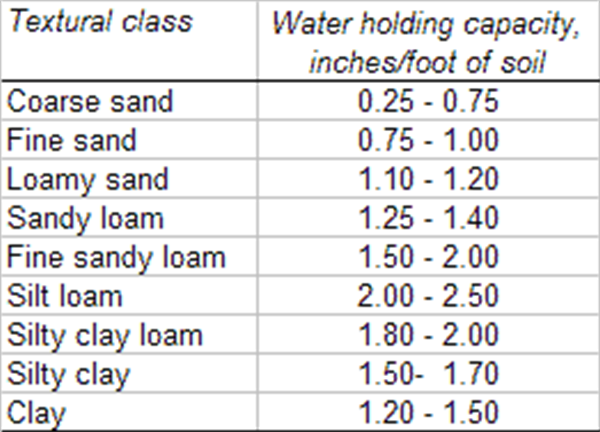
Table 1. Soil texture and water holding capacity.

Figure 1. Soil volume, soil texture, and water holding capacity relationships.

Figure 2. Soil water content relationship to plant-available water.
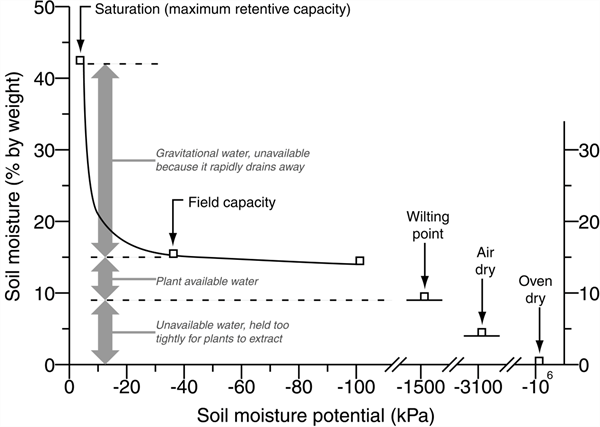
Figure 3. Soil moisture classes and important points on the soil moisture relationship curve. (Kansas State University Agronomy Department, Soil Laboratory Manual, soil and water relationships.)
References:
Allen, R. G., Pereira, L. S., Raes, D., & Smith, M. (1998). Crop evapotranspiration-Guidelines for computing crop water requirements-FAO Irrigation and drainage paper 56. FAO, Rome, 300(9), D05109.
Cotton Incorporated. Sensor-Based Scheduling. Retrieved from
http://www.cottoninc.com/fiber/AgriculturalDisciplines/Engineering/Irrigation-Management/Sensor-Based-Scheduling/
Datta, S., S. Taghvaeian, and J. Stivers. 2017. Understanding Soil Water Content and Thresholds for Irrigation Management. Oklahoma Cooperative Extension Service, BAE-1537
Hanson, B., Orloff, S., & Peters, D. (2000). Monitoring soil moisture helps refine irrigation management. California Agriculture, 54(3), 38-42.
Kansas State University Agronomy Department, Soil Laboratory Manual, soil and water relationships. https://kstatelibraries.pressbooks.pub/soilslabmanual/chapter/soil-and-water-relationships/
Ratliff, L., Ritchie, J., & Cassel, D. (1983). Field-measured limits of soil water availability as related to laboratory-measured properties. Soil Science Society of America Journal, 47(4), 770-775.
I hope you are frolicking in the fields of wildflowers picking the prettiest bugs.
I was scheduled to interview for plant pathologist position at Yuma on October 18, 2019. Few weeks before that date, I emailed Dr. Palumbo asking about the agriculture system in Yuma and what will be expected of me. He sent me every information that one can think of, which at the time I thought oh how nice!
When I started the position here and saw how much he does and how much busy he stays, I was eternally grateful of the time he took to provide me all the information, especially to someone he did not know at all.
Fast forward to first month at my job someone told me that the community wants me to be the Palumbo of Plant Pathology and I remember thinking what a big thing to ask..
He was my next-door mentor, and I would stop by with questions all the time especially after passing of my predecessor Dr. Matheron. Dr. Palumbo was always there to answer any question, gave me that little boost I needed, a little courage to write that email I needed to write, a rigid answer to stand my ground if needed. And not to mention the plant diagnosis. When the submitted samples did not look like a pathogen, taking samples to his office where he would look for insects with his little handheld lenses was one of my favorite times.
I also got to work with him in couple of projects, and he would tell me “call me John”. Uhh no, that was never going to happen.. until my last interaction with him, I would fluster when I talked to him, I would get nervous to have one of my idols listening to ME? Most times, I would forget what I was going to ask but at the same time be incredibly flabbergasted by the fact that I get to work next to this legend of a man, and get his opinions about pest management. Though I really did not like giving talks after him, as honestly, I would have nothing to offer after he has talked. Every time he waved at me in a meeting, I would blush and keep smiling for minutes, and I always knew I will forever be a fangirl..
Until we meet again.
At last week’s 2nd AgTech Field Demo: Automated Weeding Technologies, there were some practice fields that had extremely high weed pressure. It was a unique situation and company reps from the three commercial automated in-row companies participating in the Field Day wanted to test their machines to see how they would perform. We were all impressed at how well they worked in the challenging conditions –large weeds, high density (Fig. 1). Some crop plants were mistakenly removed, but not many. They also performed well in celery plots that were similarly heavily infested with weeds (Fig. 2).
It was readily apparent that image-based crop/weed differentiation using artificial intelligence and pattern recognition algorithms has come a long way in the last several years. In the past, when leaves of adjacent plants overlapped or crop plants and weeds were similarly sized, automated weeding machine imaging systems were not able to reliably detect crop plants and weeding performance was poor.
Our studies on automated in-row weeding machine performance (Lati et al., 2016) have confirmed the logical result that labor savings for follow up hand weeding operations are significant in fields where weed pressure is high as compared to where it is low. If you have a heavily infested field, it might be worth investigating the use of an automated in-row weeding machine. I was impressed at how well today’s machines work.
References
Lati, R.N, Siemens, M.C., Rachuy, J.S. & Fennimore, S.A. (2016). Intrarow Weed Removal in Broccoli and Transplanted Lettuce with an Intelligent Cultivator. Weed Technology, 30(3), 655-663.

Fig. 1. Representative image of cultivating performance of three commercial
automated in-row weeding machines (right) operated in heavily weed infested
lettuce (left). Photo taken 5 days after treatment. Machines included Stout AgTech
Smart Cultivator, FarmWise Titan FT-35 and K.U.L.T. Kress iSelect.

Fig. 2. Celery cultivated using an automated in-row weeding machine (left) and a standard cultivator (right).
It is much easier to kill weeds when there is no crop in the field and now is a good time to reduce the weed seed bank of weeds in fallow fields. It is an especially valuable time to reduce the population of perennial weeds, like nutsedge, that will continue to get worse every year until the field is almost unfarmable for many crops. Weed seeds are buried at variable depths in the soil, some have hard seed coats and there are other variables that cause them to germinate over a long period of time. If they all came up at the same time they would be much easier to control. It takes time, therefore, to repeatedly irrigate, germinate and kill weeds with either tillage or herbicides. We have conducted trials that indicate that in most years summer annual weeds begin to germinate in February, reach a peak in June but continue to germinate into October. Perennial weeds often have to be actively growing to be adequately controlled and now is a good time to get started.
Tillage Repeated germination and tillage of small annual weeds can be very effective at reducing the weed seed bank. It can also spread weeds in some situations. Proper timing of tillage to kill weeds can be important with some species. Succulent weeds like purslane can survive for several days after cultivation or hoeing. They can reroot at the nodes and continue to grow if they are allowed to get too big before they are uprooted. Growers sometimes allow early emerging weeds to get fairly big in an effort to germinate as many seeds as possible. Incorporating big weeds can temporarily build up large amounts of organic matter into the soil and have a negative effect on some preemergent herbicides used in vegetables. Many of the root and shoot inhibitor herbicides like Trifluaralin, Pendimethalin, Benefin, DCPA and others can bind to organic matter and be less available to kill weeds.
Tillage has the opposite effect on perennial weeds such as nutsedge and bermudagrass than it has on annual weeds. These weeds are spread vegetatively and repeatedly irrigating and tilling them will spread rather than kill them.
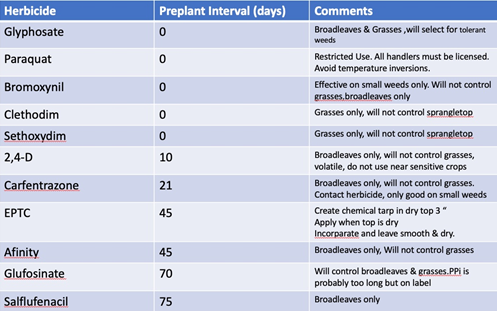
Herbicides Both contact and systemic herbicides are used during fallow periods to control weeds. The contact herbicides include Paraquat (Gramoxone, Firestorm), Carfentrazone (Aim, Shark), Pyraflufen (ET), Pelegonic Acid (Scythe) and others. Some of the advantages of these are that they are quick and have no soil residual allowing crops to be planted soon after application. Disadvantages are that they are effective primarily only on small weeds. The highest labelled rates of Paraquat is effective, however, on some big weeds.
The most commonly used systemic herbicide for fallow ground is Glyphosate. Timing is especially important when using glyphosate to control perennial weeds. Glyphosate moves both up and down from the foliage through plants. More of it moves down into the reproductive parts of perennial weeds late in the season. Late season applications will work better on perennial weeds. It is broad spectrum and has no soil residual except on very course soils. Many of the systemic herbicides registered for fallow use, such as Oxyfluorfen (Goal, Galigen) or EPTC (Eptam) require at least 90 days before planting many vegetable crops. If done correctly, Eptam can be very effective in controlling nutsedge during summer fallow. It is sensitive to use for fallow weed control and application procedure is important. Eptam is extremely volatile and can be leached or evaporated when it contacts water. It works best when incorporated into dry soil to create a chemical tarp in the top 2 to 6 inches. The best technique is to irrigate and cultivate or spray the weeds with glyphosate when dry. One half gallon of Eptam should the be incorporated into the dry surface and left smooth. Avoid getting the field wet. The longer it can be left the better. Preferably at least 30 days. Longer if possible. Some people have chemigated the Eptam on and had fair control. I am surprised that this works but higher rates than 0.5 gallon have been used which my offset much of what is lost in the water. A couple good irrigations will be necessary to remove the residual Eptam when it get warmer and before planting vegetables
Only the fumigants kill weed seeds. These include Chloropicrin, Methyl Bromide, Metam Sodium, Dazomet, Telone and others. Most preemergent herbicides only work after the seed has germinated. Preemergent herbicides are often used for fallow weed control only when at least 30 -45 days or longer are available. Fumigants are expensive, can be difficult to use and are often used for disease or nematode control with the added benefit of controlling weeds.
Soil solarization and flooding have become increasingly popular in recent years as techniques to control pests during summer fallow. Few regions are as well suited for these techniques as the low desert. They are used primarily to control diseases but have the benefit of controlling some summer annual weeds as well.