
Arizona agriculture utilizes ~ 70% of the water in this state and generates a strong and productive industry. Arizona agriculture generates more than $23B in sales as well as directly and indirectly supporting more than 138,000 Arizona jobs and employing more than 162,000 unique workers. Arizona ranks among leading states in the production of lettuce, spinach, broccoli, cauliflower, cantaloupe, honeydew melons, durum wheat, and other commodities. Arizona is an important area for the seed production of many crops that are used across the U.S. and worldwide. Many Arizona counties rank in the top 1% of all U.S. counties in terms of crop and livestock production (Murphree, 2018).
In response to the Colorado River (CR) water shortage and the current reductions in CR allocations to Arizona via the Central Arizona Project (CAP), which is primarily impacting agricultural irrigation districts in central Arizona, there is an increasing level of scrutiny on agricultural uses of Arizona water. This of course is accentuated with the recognition that agriculture utilizes ~ 70% of the Arizona water supply.
In the irrigation districts along the mainstem of the CR, there is a common adage of “First in use, first in right.” This is a fundamental aspect of the “law of the river”, which is an amalgam of the various laws, agreements, and rulings on the governance of CR water. Therefore, it is important for us to consider and prepare the positive case that can be made for the good stewardship of water resources provided by Arizona agriculture.
One common area of criticism that is directed towards Arizona crop production systems, is the use of surface and flood irrigation systems. The alternative irrigation methods that are commonly advocated for use instead of flood irrigation are methods such as drip irrigation, micro-irrigation systems, sprinklers, etc. Each of these are good irrigation methods and advantageous under the appropriate conditions. However, a good case can be made for the very efficient use of flood irrigation systems, particularly with high-flow turnouts and dead level (or very nearly so) basins for irrigation. When properly managed, these types of flood irrigation systems can be very efficient.
When we know the area to be irrigated, the flow rate of water in the irrigation delivery ditch, and the amount of water needed; then we can determine the proper time or duration for an irrigation event. If we can get fast and uniform coverage of the field to be irrigated, apply the proper volume of water to replenish the plant-available water supply to the soil, then cut off the flow of irrigation water into the field; we can do a very good job of delivery for high water-use efficiency.
To facilitate the process of managing individual irrigations for optimum efficiency, the Irrigator’s Equation can be used to estimate the depth of water applied or time (duration) of an irrigation event.
Q x t = d x A
Where: Q = the flow rate, in cubic feet per second (cfs);
t = the set time or total time of irrigation (hours);
d = the depth of water applied (inches) and
A = the area irrigated (acres).
With an understanding of the dominant soil type in the field being irrigated and the level of soil-water depletion at the time of irrigation, we can estimate the amount or depth of water needed to replenish the soil profile of plant-available water to support the crop and prevent water stress.
In managing crop fields and irrigations, we recognize that soil textures vary in terms of water holding capacities and it is important to understand the dominant soil textures in the field, not only on the surface but also through the depths of the soil profile through the effective rooting depth of the crop, Tables 1 & 2.
Collectively, we can manage surface or flood irrigation systems efficiently. In the crop production arena, it is important to communicate these points effectively.
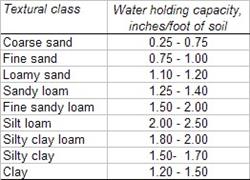
Table 1. Soil texture and water holding capacity.
2. Depths to which the roots of mature crops will deplete the available water supply when grown in a deep permeable, well-drained soil under average conditions.
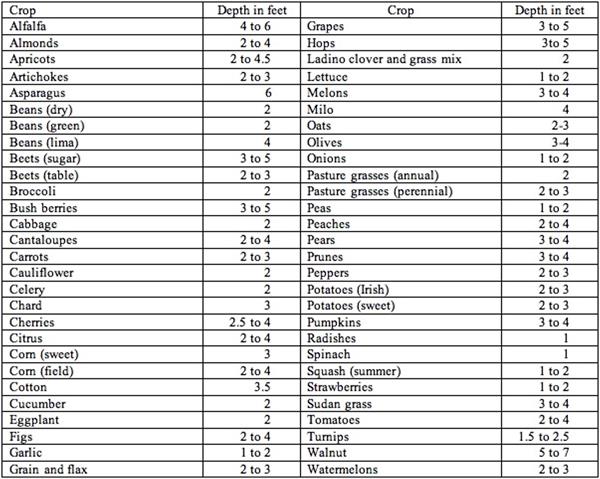
Source: Chapter 11, "Sprinkler Irrigation," Section 15, Natural Resources Conservation Service National Engineering Handbook
References:
Murphree, J. 2018. Arizona Agriculture is 23 Billion Dollars Beautiful, Arizona Farm Bureau.
https://www.azfb.org/Article/Arizona-Agriculture-is-23-Billion-Dollars-Beautiful
I hope you are frolicking in the fields of wildflowers picking the prettiest bugs.
I was scheduled to interview for plant pathologist position at Yuma on October 18, 2019. Few weeks before that date, I emailed Dr. Palumbo asking about the agriculture system in Yuma and what will be expected of me. He sent me every information that one can think of, which at the time I thought oh how nice!
When I started the position here and saw how much he does and how much busy he stays, I was eternally grateful of the time he took to provide me all the information, especially to someone he did not know at all.
Fast forward to first month at my job someone told me that the community wants me to be the Palumbo of Plant Pathology and I remember thinking what a big thing to ask..
He was my next-door mentor, and I would stop by with questions all the time especially after passing of my predecessor Dr. Matheron. Dr. Palumbo was always there to answer any question, gave me that little boost I needed, a little courage to write that email I needed to write, a rigid answer to stand my ground if needed. And not to mention the plant diagnosis. When the submitted samples did not look like a pathogen, taking samples to his office where he would look for insects with his little handheld lenses was one of my favorite times.
I also got to work with him in couple of projects, and he would tell me “call me John”. Uhh no, that was never going to happen.. until my last interaction with him, I would fluster when I talked to him, I would get nervous to have one of my idols listening to ME? Most times, I would forget what I was going to ask but at the same time be incredibly flabbergasted by the fact that I get to work next to this legend of a man, and get his opinions about pest management. Though I really did not like giving talks after him, as honestly, I would have nothing to offer after he has talked. Every time he waved at me in a meeting, I would blush and keep smiling for minutes, and I always knew I will forever be a fangirl..
Until we meet again.
It’s September and planting season is underway. When planting lettuce, uniform seed spacing is critical for efficient, economical crop thinning. Seedlings spaced too close together, commonly referred to as “doubles”, are difficult and time consuming to remove by hand. Automated thinning machines which intermittently deliver an herbicidal solution to thin excessive plants often consider doubles as a single plant. In such instances, both seedlings are left in the field (Fig. 1). This results in increased labor costs during the subsequent hand weeding operation where weeds and doubles are removed.
Several years ago, we conducted trials examining the influence of planter travel speed on seed spacing uniformity (Siemens and Gayler, 2016). In the study, a Stanhay 785 Singulaire vacuum planter was tested at travel speeds of 1.0, 1.5, 2.0 and 2.5 mph on shaped beds at the Yuma Agricultural Center. The results showed that the percentage of “difficult to thin” spacings, defined as doubles and seeds spaced less than 1 1/8” apart, increased from about 5% to 10% as speed increased from 1.0 mph to 2.5 mph (Fig. 2) . Similarly, the percentage of seeds “precisely placed” within 0.5” of the target location (i.e., 2.0 ± 0.5”) decreased from 70% to less than 45%, and the percentage of skips increased from 7% to 30%. Variability of seed spacing uniformity as measured by the coefficient of variation (COV) of seed spacings also increased. In short, planter travel speed had a significant effect on seed spacing uniformity and doubles - the higher the speed, the poorer the performance.
You may be asking what is the reason for the phenomenon observed? A logical explanation is that seeds are traveling at the speed of the planter when they are released and tend to “bounce and roll” in the direction of travel when they hit the soil surface. Thus, the higher the travel speed, the further seeds bounce and roll resulting in increased seed spacing variability. Other factors such as bed condition (i.e., cloddy vs well tilled) can also affect the amount of bounce and roll and cause poor seed placement and closely spaced seedlings that are difficult to thin (Fig. 3).
If you would like more information on how to accurately assess and analyze planter performance or are interested in conducting trials to determine how planter speed is affecting the percentage of doubles in your field conditions, please feel free to contact me.
References
Siemens, M.C., & Gayler, R.R. (2016). Improving seed spacing uniformity of precision vegetable planters. Appl. Eng. Agric., 32(5), 579-587.

Fig. 1. Lettuce seedlings intermittingly sprayed with an herbicidal solution by
automated thinning machine for purpose of thinning excess seedlings. Examples
of closely spaced seedlings, “doubles”, that are not sprayed and must be
subsequently thinned by hand (circled).
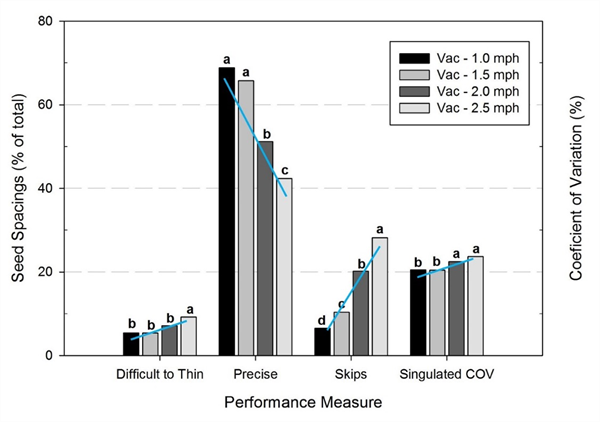
Fig. 2. Seeding performance of a vegetable planter sowing lettuce when
operated at four travel speeds.

Fig. 3. Poor seed spacing uniformity, possibly due to cloddy soil
conditions causing excessive seed bounce and roll.
Pigweed Identification
Pigweeds are some of the most common summer annual broadleaf weeds in the low deserts. Although they are often lumped together, there are 4 different species of pigweed that are common here and more than 10 species that occur as weeds in California and Arizona. Their growth habits and response to herbicides are similar. It is easy to identify them by physical characteristics but one species of pigweed can hybridize with another and become less distinguishable.
Palmer Amaranth (Amaranthus palmeri) is probably the most common pigweed species found in this region. It is very aggressive and fast growing and can become 6 feet tall or higher if uncontrolled. It has one thick stem and several lateral branches. The leaves are lance shaped, hairless and have distinctive white veins on the underside. It has flowering tassels that become stiff and spiny. This species has become resistant to Glyphosate in many parts of the county.
Redroot Pigweed (Amaranthus retroflexus) is probably the second most common pigweed species. It is shorter and the seed heads are smaller, in clusters and have stiff spine-like scales. It has leaf hairs on the margins and the veins are often reddish. The lower stems are often reddish. This species will hybridize with Palmer Amaranth and become less distinguishable.
Tumble Pigweed (Amaranthus albus) is very different from Palmers or Redroot. It grows lower to the ground and has many branches that turn upright. The leaves are much smaller and narrower. The numerous stems are light green rather than red. The seed heads are small, spiny and at the base of the leaves rather than in long terminal spikes. When mature, the branches are sticky, stiff bristles that break off at the ground and tumble with the wind.
Prostrate Pigweed (Amaranthus blitoides) is very similar to Tumble Pigweed but the stems are more prostrate, grow close to the ground and form mats. The stems and leaves are smaller and reddish rather than light green.


