
In Arizona we tend to focus on the leafy green vegetable produce industry when addressing Arizona vegetable crop production. That is quite understandable given the size, scope, and impact of the leafy green vegetable industry based in the lower Colorado River Valley in the Yuma region.
As the leafy green vegetable industry is getting started and building in the early months of the fall, chiles, another important Arizona vegetable crop and one of my favorites, is maturing and advancing in the later stages of the production season in the higher elevation valleys of southeastern Arizona, primarily Cochise County.
We commonly refer to the Southwestern (SW) Chile Belt extending from southeast Arizona, across southern New Mexico, into far-west Texas, and northern Chihuahua, Mexico. The SW Chile Belt is dominated by production the New Mexico-type chile, also commonly referred to as “Hatch chile”. The 2021 acreage across the SW Chile Belt consists of approximately 7-8,000 acres in NM, 3-4,000 in TX, approximately 90,000 acres in Chihuahua, and about 300-500 acres in Arizona. The Curry Chile Seed Company, based in Pearce, AZ, provides the seed for >90% of the total green chile acreage across the SW Chile Belt.
Chile peppers (Capsicum species) are among the first crops domesticated in the Western Hemisphere about 10,000 BCE (Perry et al., 2007). The Capsicum genus became important to people and as result, five different Capsicum species were independently domesticated in various regions of the Americas (Bosland &Votava, 2012). Early domestication of chile peppers by indigenous peoples was commonly driven for use as medicinal plants. Due to their flavor and heat characteristics, chile peppers are a food ingredient that is popular in many parts of the world, including Latin American, African, and Asian cuisines. Chiles have been increasingly important to the U.S. and European food industries, particularly as these populations become more familiar with chile (Guzman and Bosland, 2017).
There are five domesticated species of chile peppers. 1) Capsicum annuum is probably the most common to us and it includes many common varieties such as bell peppers, wax, cayenne, jalapeños, Thai peppers, chiltepin, and all forms of New Mexico chile. 2) Capsicum frutescens includes malagueta, tabasco, piri piri, and Malawian Kambuzi. 3)Capsicum chinense includes what many consider the hottest peppers such as the naga, habanero, Datil, and Scotch bonnet. 4) Capsicum pubescens includes the South American rocoto peppers. 5) Capsicum baccatum includes the South American aji peppers.
The Capsicum annuum species is the most common group of chiles that we encounter and there are at least 14 very different pod types in this single species that includes: New Mexico (aka Anaheim), bell peppers, cayennes, jalapeños, paprika, serrano, pequin, pimiento, yellow wax, tomato, cherry, cascabel, ancho (mulato, pasilla), and guajillo (Guzman and Bosland, 2017).
The source of heat in a chile comes from the capsaicin glands that run down the length of the placenta of the chile fruit. If we look at a longitudinal cross section of a chile pod (Figure 1), we can identify the placenta, the seeds, and the location of the capsaicin glands. The seeds are commonly mistaken as the source of the heat in a chile pod. This is due to the close proximity of the seeds to the placenta and capsaicin glands. The extent of the capsaicin glands is a function of genetics and in the field we know the classification of mild, medium, or hot varieties. Unfortunately, many commercial vendors do not segregate by variety and they just dump them all together, so we end up getting a rather random selection in the store.
The intensity of the "heat" of chile peppers is commonly reported in Scoville heat units (SHU). Originally, it was a measure of the dilution of an amount of chile extract added to sugar syrup before its heat is undetectable to a panel of tasters, or the more it has to be diluted to be undetectable. Thus, the more powerful the variety, the higher the rating. The modern method of determining capsaicin content is by use of a quantitative analysis of SHU using high-performance liquid chromatography (HPLC) to directly measure the total capsaicinoid content of a chile pepper sample. Pure capsaicin is a hydrophobic, colorless, odorless, and crystalline-to-waxy solid at room temperature, and measures 16,000,000 SHU (Guzman and Bosland, 2017).
Most of the commercial green chiles go into canning and food product development, e.g. salsas, etc. The varieties grown for red chile types are produced primarily for the dry chile powder. Red chiles represent the largest section of the SW and global chile industry.
References:
Bosland, P.W., E.J. Votava, and E.M. Votava. 2012. Peppers: Vegetable and spice capsicums. Wallingford, U.K.: CAB Intl.
Guzmán, I. and P.W. Bosland. Sensory properties of chile pepper heat - and its importance to food quality and cultural preference. Appetite, 2017 Oct 1;117:186-190.
doi: 10.1016/j.appet.2017.06.026.
Perry, L., Dickau, R., Zarrillo, S., Holst, I., Pearsall, D. M., Piperno, D. R., et al. (2007). Starch fossils and the domestication and dispersal of chili peppers (Capsicum spp. L.) in the Americas. Science, 315, 986-988.
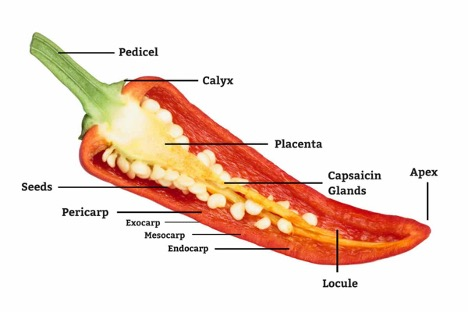
Figure 1. Chile pod anatomy.
Hi, I’m Chris, and I’m thrilled to be stepping into the role of extension associate for plant pathology through The University of Arizona Cooperative Extension in Yuma County. I recently earned my Ph.D. in plant pathology from Purdue University in Indiana where my research focused on soybean seedling disease caused by Fusarium and Pythium. There, I discovered and characterized some of the first genetic resources available for improving innate host resistance and genetic control to two major pathogens causing this disease in soybean across the Midwest.
I was originally born and raised in Phoenix, so coming back to Arizona and getting the chance to apply my education while helping the community I was shaped by is a dream come true. I have a passion for plant disease research, especially when it comes to exploring how plant-pathogen interactions and genetics can be used to develop practical, empirically based disease control strategies. Let’s face it, fungicide resistance continues to emerge, yesterday’s resistant varieties grow more vulnerable every season, and the battle against plant pathogens in our fields is ongoing. But I firmly believe that when the enemy evolves, so can we.
To that end I am proud to be establishing my research program in Yuma where I will remain dedicated to improving the agricultural community’s disease management options and tackling crop health challenges. I am based out of the Yuma Agricultural Center and will continue to run the plant health diagnostic clinic located there.
Please drop off or send disease samples for diagnosis to:
Yuma Plant Health Clinic
6425 W 8th Street
Yuma, AZ 85364
If you are shipping samples, please remember to include the USDA APHIS permit for moving plant samples.
You can contact me at:
Email: cdetranaltes@arizona.edu
Cell: 602-689-7328
Office: 928-782-5879
Today's video is a demonstration of a spray assembly delivering herbicidal spray (blue dye) to spot spray targeted weeds. This devise can be integrated with an imaging system for precision, automated / robotic weed control.
CHECK IT OUT!
Herbicide resistant weeds have received a lot of attention in recent years. It is often misunderstood. Three of the most misunderstood concepts regarding herbicide resistance are: 1- Weed tolerance and weed selection are not resistance,2- Weed resistance is not universal and does not affect every weed of a certain species from field to field or within a field and weed resistance often takes much longer than insect resistance that is more common and occurs faster.
No Herbicide controls all weeds. Those weeds that are not controlled are tolerant. They never were controlled by that particular herbicide and they are often selected for and become more prevalent over time if the same herbicide is used. Resistant weeds, on the other hand, were controlled at one time by a particular herbicide and have naturally developed a trait that stops the herbicide from working. These resistant weeds survive from generation to generation and become more prevalent over time.
Weed resistance does not occur in all weeds in a field at the same time. It can be just one plant of trillions in a field. As this plant survives the herbicide and goes to seed it becomes more widespread in the field and in other fields. We conducted a trial in Parker last year where sprangletop survived Glyphosate in one field and was killed by the same treatment down the road. If your neighbor has resistant weeds it doesn’t mean that you do too.
Lastly, insect resistance to insecticides has occurred in this region for many years and was the first exposure that many pest control advisers and growers had to pesticide resistance. The principals are the same although insects generally produce multiple generations per season and mutations that facilitate resistance occur faster than for weeds. Annual weeds often produce only one or two generations per season and resistance takes much longer.
This time of year, John would often highlight Lepidopteran pests in the field and remind us of the importance of rotating insecticide modes of action. With worm pressure present in local crops, it’s a good time to revisit resistance management practices and ensure we’re protecting the effectiveness of these tools for seasons to come. For detailed guidelines, see Insecticide Resistance Management for Beet Armyworm, Cabbage Looper, and Diamondback Moth in Desert Produce Crops .
VegIPM Update Vol. 16, Num. 20
Oct. 1, 2025
Results of pheromone and sticky trap catches below!!
Corn earworm: CEW moth counts declined across all traps from last collection; average for this time of year.
Beet armyworm: BAW moth increased over the last two weeks; below average for this early produce season.
Cabbage looper: Cabbage looper counts increased in the last two collections; below average for mid-late September.
Diamondback moth: a few DBM moths were caught in the traps; consistent with previous years.
Whitefly: Adult movement decreased in most locations over the last two weeks, about average for this time of year.
Thrips: Thrips adult activity increased over the last two collections, typical for late September.
Aphids: Aphid movement absent so far; anticipate activity to pick up when winds begin blowing from N-NW.
Leafminers: Adult activity increased over the last two weeks, about average for this time of year.







