Insect Management on Desert Melons: Whiteflies 2019
In the past 25 years, the sweetpotato whitefly, Bemisia tabaci, (SWF) has shifted from a position as an occasional virus vector to being one of the primary pests in vegetables and melons in the desert. This shift in pest status is thought to have occurred due to the development of a new strain of the sweetpotato whitefly, MEAM1 (aka, B-biotype). Another Bemisia whitefly (Q biotype of B. tabaci) was discovered on ornamental plants from retail nurseries in Arizona and California several years ago. This new strain is believed to be highly resistant to number of key insecticide chemistries but, to date, has not been detected on field grown vegetables or melons in the desert.
Distribution and Host Range.
SWF is found on vegetables and melons through the southern U.S and is thought to be native to Mediterranean growing regions. It has a very broad host range with over 60 host plants being reported in the desert alone. It will readily colonize and feed on most, if not all, leafy vegetables, cole crops and melons. Commonly grown field crops also serve as important hosts, including cotton, alfalfa, and sugarbeets. Weeds such groundcherry, purslane, malva and wild lettuce are readily colonized. Ornamentals such as lantana, roses, bougainvillea and hibiscus will also sustain large numbers of SWF in urban areas adjacent to crop fields.
Description and Seasonal Development.
SWF adults are not strong fliers and typically move short distances in search of host plants. They usually move downwind in the early morning, but following certain storm conditions, can be transported long distances. Once SWF find a suitable host, minute, oblong eggs are deposited mainly on the underside of leaves (Plate 1). Near hatching, the egg will darken and hatches in 2 to 5 days. All nymphal instars are translucent, and somewhat shiny in appearance. Only the first instar “crawler” is mobile. Crawlers are yellowish in color and are oval and flattened in appearance (Plates 2- 4). They will move about until they locate a suitable feeding site. Once they locate an acceptable feeding site, they become immobile and remain so through four nymphal instars. They feed by inserting their mouthparts into the vein and extracting phloem sap. Late third and fourth instar nymphs have distinctive red eye spots and are termed red-eyed nymphs. At the end of the fourth instar they enter what is called the pupal stage, but the nymph still feeds. Their pupal cases are dome shaped and oval. Total pre-adult development time averages 15-18 days under optimal temperatures (86 °F) and host conditions. SWF adults are white, with a yellow abdomen and red eyes. They begin oviposition within 5 days after emergence, can live for up to 20 days and may produce about as many as 300 eggs. SPWF generally thrive in hot, dry weather and during the summer can complete a generation in less than 20 days. In lower desert areas, up to 12-13 generations may occur annually. Rainfall can be detrimental to SWF, particularly heavy monsoon thunderstorms.
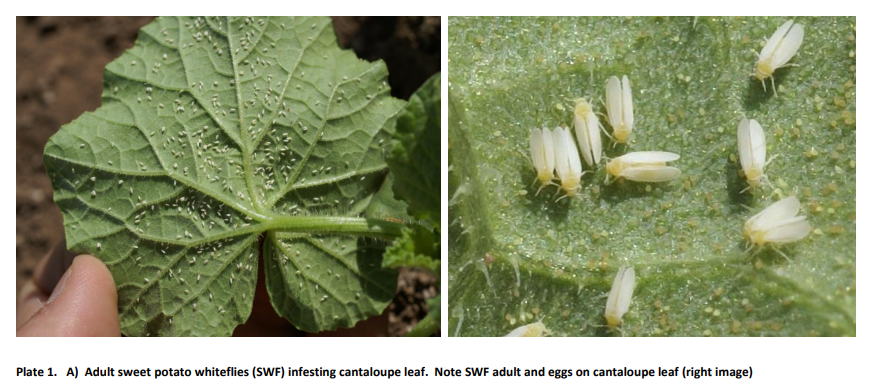
Economic Damage.
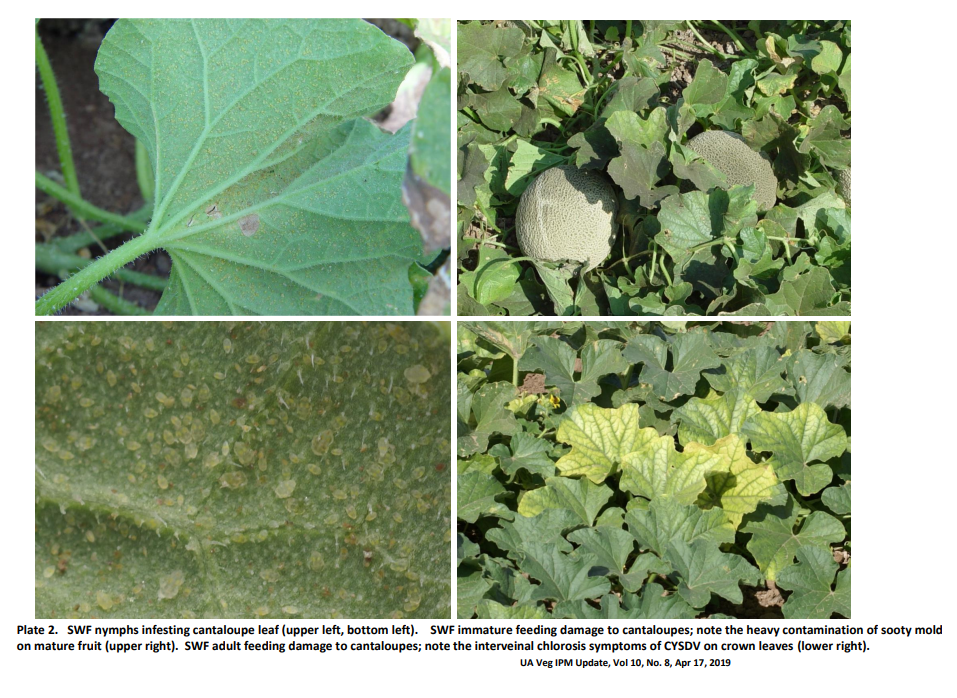
In general, SPWF cause crop damage in three ways. First, adults and nymphs feed on leaves by inserting their sucking/piercing mouthparts into vascular phloem tissue and extracting plant juices (carbohydrates and amino acids). This can result in leaf discoloration and yellowing. Under heavy feeding pressure wilting, defoliation and severe growth reduction may occur. Systemic feeding effects (toxic secretions by nymphs) can occur on young, un-infested leaves by SWF feeding on older infested leaves. In addition, SWF also causes indirect damage to plants by transmitting plant viruses and are known to transmit over 60 plant viruses. Finally, SWF can directly damage crops by contamination of the harvested product. Adult and nymphs excrete large amounts water and sugar in the form of honeydew which accumulates on the upper surface of leaves and fruit. This sticky excrement allows for sooty mold growth that when excessive can inhibit leaf photosynthetic activity and render fruit unmarketable.
Although the SWF has a wide host range, one of its most preferred hosts is melons. It has become a serious pest on melons because of its high reproductive capability, wide host range, high rate of feeding, exudation of sticky honeydew and habit of feeding on the undersides of leaves where they are protected from insecticide sprays. Adults and nymphs feed on melon leaves by inserting their tubular mouthparts into vascular tissue and extracting plant assimilates (carbohydrates and amino acids). They also injure developing plants by destroying chlorophyll and reducing the plants photosynthetic activity. Heavy populations on young plants can cause desiccation of leaves and plant death.
SWF populations cause serious economic damage to melons crops by reducing fruit quantity and size. Fruit quality is also impacted by the lowering of soluble sugars in the fruit and by the contamination of fruit with honeydew which gives rise to sooty mold (Plate 3 C). In addition, heavy populations stress plants and lower the plants tolerance to disease infections from organisms such as Monosporascus cannonballus. SWF adults can also transmit important viruses on melons. A new virus that showed up in 2008 in Arizona can be very damaging; Cucurbit Yellows Stunting Disorder Virus (CYSDV) (Plate 3D). This pathogens is more severe in fall melons associated with higher temperatures and heavy adult infestations.
Management of SWF
SWF populations on a given crop during the season will vary from year to year. Their abundance is influenced by several naturally occurring factors within the cropping system as well as management practices employed by growers. Weather patterns and seasonal temperatures probably have the largest natural influence in regulating abundance and population growth during the season. Natural enemies also impact SWF populations in crop and non-crop hosts. However, these factors alone are generally not capable of preventing economic damage from occurring. Rather, several fundamental management approaches in association with naturally occurring factors have allowed growers to economically produce vegetables and melons in the desert over the past several years. Preventing SWF populations from colonizing plants is the key to successful management. This is achieved primarily by preventing the establishment of immature SWF, often through management of adult populations. If adults are allowed to oviposit on the undersurfaces of leaves, it becomes very difficult and often very expensive to avoid damage from the feeding nymphs. Presently, several effective management practices and control tactics are available that act against both adult and immature populations. In addition, because whitefly adults move between successive crops, management approaches are most successful when employed in all crops within the area.
Sampling/Monitoring. Yellow Sticky traps can be used to monitor adult flight activity and are particularly useful in the spring when population densities are low. Although useful in certain situations, sticky traps have not been found to accurately reflect population levels within melon fields. Sampling for adults and immature SWF should be concentrated on young leaves, whereas sampling for nymphs on older foliage. Visual observations of adults on the 5th node leaf from the main stem terminal provides the most accurate and practical method for estimating field populations. Adults should be sampled during early morning hours by carefully turning leaves before adults are active. Sampling for immatures should include visual observations of nymphal development on the underside of leaves near the crown portion of the plant. This entails using a 10X-20X hand lens to observe the presence of nymphs on the undersides of leaves. Early season sampling (planting to first fruit set) should be focused primarily on crown leaves found on the primary vine (3rd - 5 th leaf from cotyledon). From fruit set until harvest, sampling should be focused near the middle of the primary vine.
Natural / Biological Control Although many predators, parasitoids, and fungal diseases are known to attack SWF, none are known to provide adequate economic control under typical desert growing conditions. The general predators usually associated with SWF include minute pirate bugs, green lacewings , big-eyed bugs, and lady beetles. Parasitic wasps in the genera Encarsia and Eretmocerus are the most commonly found in the desert. Presently, the augmentation of natural enemies (parasitoids/predators) for control of whiteflies is not practiced in desert production. It is unlikely that the use of biological control would have much impact in fall planted crops because of the overwhelming numbers of adults that migrate during stand establishment. Although there have been several formulated foliar spray products containing fungal pathogens with efficacy against UA Veg IPM Update, Vol 10, No. 8, Apr 17, 2019 whiteflies (i.e., Beauvaria bassiana), they have had only limited success due to their requirement of high humidity for sporulation and the need for undersurface spray coverage for direct contact with developing nymphs.
Cultural Practices: There are three times during the seasonal cropping cycle when whitefly management is critical in the desert; during the winter when whitefly abundance is lowest, in late spring during the transition from melons to cotton, and in August and September during the transition between cotton or alfalfa to fall vegetable/melon plantings. These periods all involve the movement and colonization by adult populations. Consequently, cultural management practices can help to avoid or minimize problems with SWF before they have the chance to occur.
•
Crop Management. It is important to utilize optimal growing practices to avoid stressing newly emerged seedlings. This would include proper management of irrigation, plant nutrition and salinity. Experience has shown that a vigorously growing plant is better able to withstand external stresses like SWF. This is especially important for fall melons and vegetables planted a time of high temperature stress. Any additional stresses can delay growth and effect yield potential. SWF have a competitive advantage because temperature at this time of the season are often optimal for their development, whereas cool season crops like leafy vegetables and cole crops struggle to grow.
•
Crop Scheduling. Careful consideration of crop sequencing, crop placement, and planting dates can have significant impacts on adult SWF migration. When practical, it is recommended that growers avoid planting fall melons and vegetables near other significant host crops such as alfalfa and cotton. Delaying fall planting until after termination or harvest can also reduce adult migration onto seedling crops. Early planting of spring melons and uniformity of planting in an area can reduce the impact of SPWF as well.
•
Source Reduction. In preparing for the establishment of new crop planting, it is always important to be aware of adjacent crops and other natural habitat. Host-crops approaching harvest (vegetable seed, cotton, alfalfa) and weedy non-crop areas are usually the primary sources from which adult SWF migrate. Sanitation and clean culture are perhaps the most important cultural practices that can be employed on an area-wide basis. Rapid post-harvest destruction of host crops can reduce the magnitude and duration of whitefly movement in an area. It is also important to eliminate weed hosts in and around fields to be planted, particularly in the winter where they can serve as overwintering reservoirs for SWF and viruses.
•
Row covers: Fabric row covers can decrease SWF infestations of fall crops, and severity of viruses. However, deployment of these covers is often labor intensive and expensive.
Insecticidal Control
The most immediate and direct approach to control SPWF in vegetables is through the use of insecticides. Presently, growers can control whiteflies with chemicals in the following ways: a responsive approach using contact foliar applied insecticides or an insect growth regulator, a prophylactic approach using a soil applied systemic insecticide, or a combination of foliar and soil applied insecticides. These approaches are aimed at preventing adults from colonizing plants and depositing large numbers of eggs. Although mortality to nymphs from contact foliar sprays does occur, coverage with conventional application equipment is not usually adequate enough to control a large proportion of the nymph population, especially on lower leaves.
• Foliar Responsive Approach. The use of foliar insecticides to suppress adult populations is dependent on several factors. First, the residual effectiveness of foliar insecticides is related to the duration of adult migration. If adults are continually moving into a field, repeated application may be necessary to prevent significant colonization. The residual of the insecticides and the rate at which the plant grows will also affect the performance of an individual application. In addition, good spray coverage is necessary even for activity against adult populations. Combinations of pyrethroids (esfenvaluarate, bifenthrin, lamba-cyhalothrin or fenthropropin) and UA Veg IPM Update, Vol 10, No. 8, Apr 17, 2019 organopshosphates (acephate, chlorpyrifos), cabamates (Vydate, or Lannate) can provide short term residual knockdown (1-3 days) of adult populations. These products work primarily against the adults, but with thorough coverage, can kill nymphs. Neonicotinoid foliar products such as acetamidiprid (Assail) and dinotefuron (Venom/Scorpion) have translaminar activity and provide more residual (4-10 d) control of adults. The new feeding blockers (PQZ and Sefina) can provide excellent control of adults and when used in a program, can significantly suppress CYSDV. However, these products also provide good residual (11-14 d) control of nymphs on treated leaves. Exirel and Minecto Pro (cyazypyr) are also effective alternatives against both adults and nymphs (~14 d residual). They will also control leafminers, cabbage looper and beet armyworm, and Minecto Pro will provide spider mite activity due to the inclusion of abamectin. Timely application of foliar insecticides is important for control of whiteflies. It is recommended that whitefly populations be treated with insecticide if they exceed 2 adults per leaf. On fall melons, because plants are usually small when adults are migrating, a greater number of sprays are often needed. This is again dependant on the magnitude and duration of adult immigration. There are other limitations to use of foliar insecticides. First, there are a limited number of products combinations available, so rotation of chemistries is important for resistance management. Other pests such as worms and leafminers also require treatment at various times so total pest control on a fall crop can often become quite expensive. Finally, it is important to not apply these products when bees are actively working in the field.
• Soil-Applied Neonicotinoids. Presently, the use of a soil-applied treatments of imidacloprid (Admire Pro, Alias, and other generics), thiamethoxam (Platinum, Durivo) or dinotefuran (Venom) at planting is the industry standard for control of SWF in vegetables and melons. A newer compound, flupyradifurone (Sivanto) also provides excellent activity against SWF adults and CYSDV in melons. These neonicotinoid and neonicotinoid-like compounds have varying degrees of soil mobility and can differ in knockdown and residual efficacy depending on temperature, timing, placement and rate. The materials are taken up by the roots and distributed throughout the plant via xylem transport. They are active against feeding adults, and consequently, colonization by immature SWF is reduced because of the reduction in egg deposition. Crawlers are generally not affected by the compounds until they begin to feed. Second instar nymphs that do emerge usually die within a week from feeding on the treated plant. Soil applied neonicotinoids have been shown to effectively control whiteflies when injected at moderate to high rates 1.5 - 3 inches below the seed line (sub-seed furrow) just prior to planting. They are also effective at controlling whiteflies when applied immediately through low pressure drip irrigation systems. When applying through a drip system, it is important that the emitters are positioned to deliver the material at the base of the plant to optimize root uptake. Immediately after crop emergence, it is not uncommon to find adult whiteflies and eggs on treated plants, particularly on the cotyledons. This is not necessarily indicative of an insecticide or application failure, but rather a delay in uptake of the material. Once the plants reach the one to three leaf stage, activity should be evident. However, because adult SWF may continually migrate into the field, do not base control on adult densities. At thinning stage, check the first couple of basal leaves. If treated plants treated contain high numbers of large nymphs on older leaves, alternative control measures should be taken.
• Foliar Population Regulation Approach. Alternative foliar compounds are available that have selective activity, primarily against immature SWF. These include the insect growth regulators (IGR), Courier (buprofezin) and Knack pyriproxyfen) , and an IGR-like compounds Oberon (spiromesifen). Their modes of action UA Veg IPM Update, Vol 10, No. 8, Apr 17, 2019 are not neurotoxic but rather disrupt either insect hormonal or physiological functions. Courier is a chitin synthesis inhibitor that works by disrupting the molting process between developing nymphal stages and works primarily through vapor inhalation and direct contact. Knack is a juvenile hormone analog with translaminar activity that works by killing eggs and hatching crawlers, and preventing adult formation. Oberon is a lipid biosynthesis inhibitor with translaminar activity that works against all nymph instars, and has some effect on adult fecundity. These products provide effective residual (21-28 d) control of SWF nymphs when applied properly. Spray applications should be as precise as possible. Applications on spring melons should be initiated when SPWF adult exceed 2 per leaf or when more than 1 red-eyed nymph per leaf can be observed on fall crops. These compounds should be applied with ground application equipment in a minimum of 20 GPA whenever possible. Directed sprays should be made to ensure maximum deposition of spray droplets on leaf surfaces. If applied correctly, 3- 4 weeks of residual control can be expected under spring growing conditions. Under higher temperatures (July-Sep), less residual can be expected. Although these compounds have not shown negative effects on pollinating honey bees, the products should be applied a night whenever possible. They are also easy on most natural enemies that feed on whitefly immatures
Consideration of Pollinators
• Because cantaloupes and watermelons are monecious, pollination by bees is essential for the production of high quality melons. Colonies of honeybees must be placed in or around fields to ensure pollen transfer from staminate to pistillate flowers. Insufficient pollination will result in misshapen melons and reduced fruit set. To produce high quality melons, it is recommended that 2-3 hives per acre be placed in each field.
• Consequently, extreme care must be taken with pesticides to prevent the destruction of honey bees. Some insecticides used in melon pest management programs are highly toxic to these pollinators. If insecticides are not applied properly when crops are flowering, bee kills can occur. Losses can result from direct sprays on bees, drift onto hives or adjacent fields, and by contamination of drinking water, pollen or nectar.
• The following practices are very important in planning pesticide applications.
1) If possible, avoid making pesticide applications when melons are in bloom.
2) If applications are necessary during bloom, apply the pesticide that is least toxic to bees and will still control the target pest.
3) Only apply insecticides during the evening (10:00 pm-3:00 am) when the bees are not actively working in the field.
4) Finally, it is a very important precaution that the beekeeper be notified before spray applications are made to blooming crops. The advanced notice allows beekeepers to take steps to move or protect their hives if necessary.

