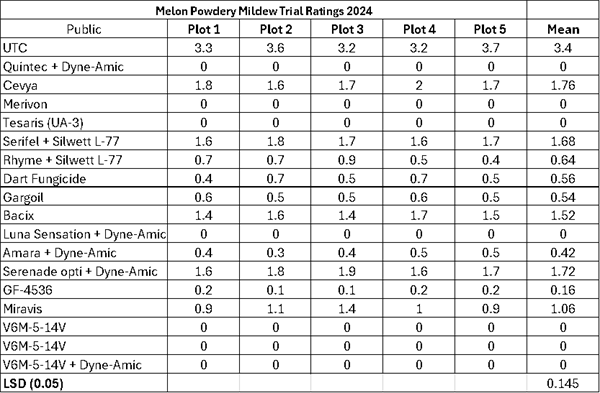
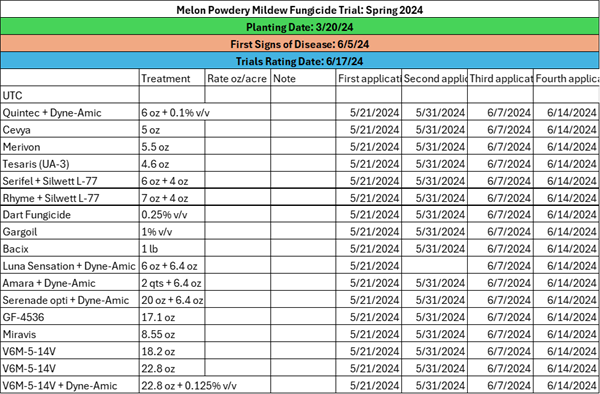
-
Apr 3, 2019Lettuce Aphids Showing Up on Late
This season has been a banner year for aphids in the desert. Aphid pressure has been steady on most crops throughout the spring. Consistent with the warm weather we have been experiencing lately, Lettuce aphids, Nasonovia ribisnigri, (aka., Red aphid) have shown up in desert lettuce again. The last time lettuce aphid was this abundant on desert produce was the spring of 2012. However, in the past couple of weeks, I've identified lettuce aphid appearing on both head lettuce and romaine in Roll and Wellton. I’ve also had reports of the aphid showing up in the Imperial Valley. On the Yuma Ag Center, we began seeing lettuce aphid a couple of weeks ago on untreated lettuce, and they have recently built up to high numbers. Previous experience has taught us that daytime high temperatures in the 80's are ideal for population growth. A few things to remember about lettuce aphids relative to the other aphids we commonly see. First, the immature nymphs are small and have a red appearance, whereas the apterous adults are typically a large brown colored aphid with dark bands running across the abdomen. Second, among the crops we grow locally, lettuce aphids are only found on the lettuce types (Lactuca spp.). Lettuce aphids prefer to colonize the terminal growth, and can often be found in heads or hearts, whereas green peach aphids are often found on the frame leaves in high numbers before moving into the heads. Sampling should be focused in the terminal growth of young plants, and in the heads and hearts of older plants. Third, they can reproduce prolifically, producing many more winged alates than other species, which can quickly lead to widespread abundance throughout a field or a growing area. Finally, as the saying goes "he who hesitates is lost". If lettuce aphids are found on your lettuce, it is recommended that you respond quickly with an insecticide treatment. The product of choice is Movento at 5 oz/ac. Because of its systemic activity, Movento will reach aphids in the protected terminal growth. Be sure to include a penetrating adjuvant for best results at a rate of at least 0.25% v/v. It normally requires 7-10 days of activity before significant reduction in the infestation is observed. For more information on lettuce aphid please refer to Lettuce Aphid on Late Season Produce. If you’re finding lettuce aphid on organic lettuce, you’re out of luck; none of the registered biopesticides will control this aphid.

 To contact John Palumbo go to: jpalumbo@ag.Arizona.edu
To contact John Palumbo go to: jpalumbo@ag.Arizona.edu