
What you should know about N95 respirators and face masks
Shujuan Li1, Dawn H. Gouge1, Jennifer Weber2, Stephen A. Kells3, Janet A. Hurley4,
Alfred J. Fournier1, Shaku Nair1, Kaci Buhl5
1. Department of Entomology, University of Arizona.
2. Maricopa County Cooperative Extension, University of Arizona.
3. Department of Entomology, University of Minnesota.
4. Texas A&M AgriLife Extension.
5. Department of Environmental & Molecular Toxicology, Oregon State University.
The use of personal protective equipment is one part of a risk reduction strategy.
It is very important to use a combination of tactics to protect yourself and others from harm.
Personal protective equipment (PPE) refers to protective devices worn to minimize exposure to hazards that cause injuries and illnesses. PPE acts as a physical barrier preventing the wearer from exposure to chemicals (e.g., pesticides), radiation (e.g., radon gas), electricity (e.g., live electrical wires), mechanical dangers (e.g., machinery that can cause crushing or entanglement), physical dangers (e.g., loud sounds, airborne dust), biohazards including disease causing pathogens (e.g., pathogenic bacteria and viruses) or waste contaminated with potentially infectious materials (e.g., blood, fecal material).
PPE includes job specific gloves, safety glasses, shoes, earplugs, hard hats, respirators, vests, splash suits, aprons, coveralls, jackets, lab coats, surgical gowns, and full-body suits, to list a few common items. Each item is precisely designed to protect the wearer from very specific hazards.
PPE is sometimes used to prevent hazardous exposure from vulnerable people. For example, disposable surgical scrubs including gowns, gloves, caps, shoe covers, masks, and protective goggles are worn to protect patients from microorganisms in and on the body of the surgical team, and to protect the surgical team from the patient's blood and bodily fluids during surgery or treatment.
The use of PPE may be legally required in work environments (e.g., hardhats in building sites), or legally required in order to use certain tools or products (e.g., pesticide product labels indicate federally required PPE for applicators).
PPE is designed to protect vulnerable body parts from specific hazards, for example, chemically resistant gloves are used to protect the skin on your hands from pesticide exposure, while an N95 respirator protects the mouth and nose from particles including small particle aerosols and large droplets (only non-oil aerosols).
Personnel who handle pesticides are required to wear PPE listed as described on specific pesticide labels according to federal law.
There are many types of respirators; this document only addresses the N95 type.
The U.S. Food & Drug Administration states, “N95 respirators and surgical masks (face masks) are examples of PPE that are used to protect the wearer from airborne particles and from liquid contaminating the face.” The U.S. Centers for Disease Control and Prevention (CDC) - National Institute for Occupational Safety and Health (NIOSH) and the U.S. Department of Labor - Occupational Safety and Health Administration (OSHA) both regulate N95 respirators.
OSHA has issued Temporary Enforcement Guidance to Compliance Safety and Health Officers for enforcing the Respiratory Protection standard, 29 CFR § 1910.134, with regard to supply shortages of N95 filtering facepiece respirators due to the COVID-19 outbreak https://www.osha.gov/memos/2020-03-14/temporary-enforcement-guidance-healthcare-respiratory-protection-annual-fit.
Due to the COVID-19 pandemic, disposable N95 respirators and medical grade face masks (surgical face masks) are in short supply.
All N95 respirators are designed to achieve a very close facial fit around the nose and mouth. They come in different sizes, and people with facial hair may not be able to use an N95 respirator effectively if they are unable to achieve a tight seal.
Surgical N95 respirators are commonly used in healthcare settings and are a subset of N95 filtering facepiece respirators (FFRs), but these are often referred to simply as N95s.
Some pesticide labels specify several acceptable respirators, e.g., Javelin® WG (Figure 1). Please note a NIOSH TC-84A respirator is a disposable N95 respirator (Figure 2). Reusable NIOSH TC-84A respirators (Figure 3) fitted with High Efficiency (HE) filters are also an acceptable option stipulated in the same pesticide label (Figure 1).

Figure 1. Note the circled PPE requirements on this pesticide label from
Javelin® WG. BOTH respirators are listed on the same pesticide label so if a
disposable N95 is not available, the applicator can use the other type (these are available).
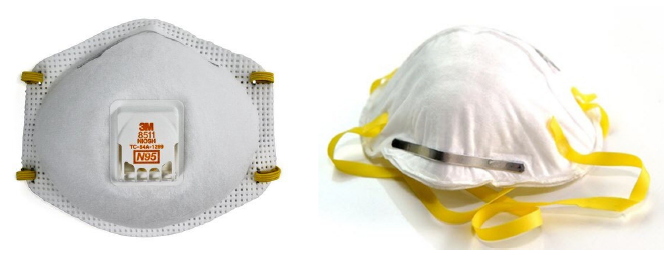
Figure 2. A NIOSH TC-84A disposable N95 respirator with exhalation valve (left), and an
N95 respirator without an exhalation valve (right).

Figure 3. An example of reusable TC-84A respirator with fitted filter.
Pest management professionals can improve their understanding of respirators by reviewing the information provided by the Pesticide Education Resources Collaborative (PERC) who have placed clear explanations on all aspects of respirator use for pesticide handlers in English and Spanish: http://pesticideresources.org/wps/definitions/respirator.html.

All PPE intended for use as medical devices must follow the Food and Drug Administration (FDA)’s regulations (https://www.fda.gov/) and should meet applicable standards for protection. N95 respirators are used in healthcare settings, often as single-use, disposable respiratory protective devices, used and worn by healthcare personnel to protect both the patient and themselves from the transfer of microorganisms, body fluids, and particulate material. Some products are approved by the National Institute for Occupational Safety and Health (NIOSH) as an N95 respirator and cleared by the FDA for medical use. The FDA has a Memorandum of Understanding (MOU) with CDC NIOSH that outlines the framework for coordination and collaboration between the FDA and NIOSH for regulation of a subset of N95 respirators for medical use.
On March 14, 2020 the U.S. Department of Labor issued temporary enforcement guidance for respirator fit-testing in healthcare settings during the COVID-19 outbreak due to supply shortages and emergency measures (https://www.osha.gov/news/newsreleases/national/03142020). Additionally, the U.S. Department of Labor’s OSHA has issued new temporary guidance (https://www.osha.gov/memos/2020-03-14/temporary-enforcement-guidance-healthcare-respiratory-protection-annual-fit) regarding the enforcement of OSHA’s Respiratory Protection Standard (https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.134). This guidance is aimed at facilitating healthcare workers’ access to N95 respiratory protection.
OSHA’s temporary suspension of annual fit testing for N95 respirators, remains in effect until further notice, and only applies to healthcare workers who have already been fit-tested. This does not apply to pesticide applicators.
Healthcare workers caring for COVID-19 patients, in-home care providers managing COVID-19 patients, and confirmed or suspected COVID-19 patients should be using N95 masks. However, due to ongoing shortages of respiratory protection, the CDC has issued interim guidance for healthcare workers allowing the use of other classes of full-face respirators (FFRs), including elastomeric half-mask and full facepiece air purifying respirators, and powered air purifying respirators when possible: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Finfection-control%2Fcontrol-recommendations.html.
CDC lists all approved models here: https://www.cdc.gov/niosh/npptl/topics/respirators/disp_part/default.html.
Currently NIOSH-certified N95 respirators that have not been cleared by the FDA can be used for respiratory protection in the healthcare settings. They reduce the exposures of healthcare personnel in a patient setting to hazardous particulates. However, it is important to note that these respirators have not been evaluated by FDA to determine whether they meet the fluid and flammability resistance as required for FDA clearance as medical devices. For this reason, they are not intended for use in exposure settings where the performance of a mask to maintain a sterile field is required.
N95s with an exhalation valve can be used in a healthcare setting when it is not important to maintain a sterile field. Exhalation valves make it easier to exhale. Respirators with exhalation valves should not be used in situations where a sterile field is required because the exhalation valve allows unfiltered exhaled air to escape into the sterile field
A face mask (Figure 4) is a loose-fitting, disposable or washable device that creates a physical barrier between the mouth and nose of the wearer and potential contaminants in the immediate environment. Not all face masks are certified as surgical face masks for use in medical environments. The most significant difference between an N95 and a loose-fitting, face mask is that the edges of the face mask are not designed to form a seal around the nose and mouth of the wearer.
Sometimes ill patients are asked to wear these devices to limit the spread of pathogen-carrying droplets produced by the wearer to others.
An infographic has been created by the CDC to help explain the difference between a surgical face mask and an N95 respirator https://www.cdc.gov/niosh/npptl/pdfs/UnderstandDifferenceInfographic-508.pdf.
If you are not a healthcare worker or a patient under care, please use face covers that are not medical grade devices while supplies are needed for medical use.

Figure 4. Disposable face masks (surgical face masks).
Since a significant number of people can carry SARS-CoV-2 (the virus that causes COVID-19) and be unaware that they are infected and shedding the virus, the CDC has issued a recommendation to cover your mouth and nose with a cloth face cover when around other people: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html.
The current CDC guidance (April 8, 2020) reads as follows:
Cover your mouth and nose with a cloth face cover when around others.
Keep yourself appraised of updated information from the CDC on How to Protect Yourself & Others: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html.
Use cloth face coverings to help slow the spread of COVID-19 (Figure 5). Face coverings will reduce the number of droplets released into the air as a person exhales, speaks, yawns, sneezes, or coughs, and will also reduce the wearer’s ability to stick their fingers in their nose or mouth. The CDC reports that a washing machine can be used to properly wash face coverings.

Figure 5. Consider making your own face masks (left) or utilize regular scarves
(right), bandanas, and other clothing (similar to ideas shown in photos).
People with chronic respiratory, cardiac, or other medical conditions that make breathing difficult should check with their healthcare provider before using an N95 respirator, as such devices can make it even more difficult for the wearer to breathe. Unfortunately, N-95s with an exhalation valve will not protect bystanders if the wearer is infected and shedding a virus.
Note that all FDA-cleared N95 respirators are labeled as "single-use" disposable devices. If your respirator gets damaged, soiled, contaminated, or if breathing becomes difficult at any time during use, you should remove the respirator, discard it properly, and put on a new one. To safely discard your N95 respirator, place it in a plastic bag and put it in the trash. Wash your hands thoroughly after handling the used respirator.
The best way to prevent airborne transmission of the SARS-CoV-2 is to use a combination of interventions, not just PPE alone.
If a pesticide handler plans to work with a pesticide requiring the use of an “N95” respirator, but is unable to acquire one, the applicator can use a respirator that exceeds the N95 ratings. The pesticide handler can look for respirators bearing the rating numbers “99” or “100”, which will filter out 99 or 99.97% of particles, exceeding the 95 rating. However, these respirators might be heavier and bulkier and contain an exhalation valve, offering no protection for bystanders from a SARS-CoV-2 infected pesticide applicator.
The letter designations “N”, “R” and “P” are also important:
An “N” rating is sufficient for COVID-19 viral capture (https://www.cdc.gov/niosh/npptl/topics/respirators/factsheets/respsars.html). When applying a pesticide or a disinfectant, always check the label and letter designation. Some disinfectant labels require a 95 rating, in which case an N, R, or P respirator will be effective. However, some pesticides have letter designations indicated and only respirators with that specific designation should be used.
Pest management professionals undertaking pest control and cleanup activities involving pests associated with human disease-causing pathogens (e.g., hantavirus is associated with deer and white-footed mice) should not work without the appropriate PPE. Choose more protection, not less and if you have questions contact 1-800-CDC-INFO (800-232-4636), TTY: 888-232-6348 or email CDC-INFO.
Now is a great time for pesticide handlers to improve their knowledge about respirator types, use and maintenance, and for pest management professionals to adopt an integrated pest management (IPM) strategy, which in many instances can reduce dependence on pesticides.
If you would like to know more about “What is Essential Pest Control Service during the Coronavirus/COVID-19 Outbreak?”, please view: http://www.stoppests.org/frequently-asked-questions/covid-19-resources/#essential
Information in this article was compiled from CDC, OSHA, FDA, EPA, and other documents related to respirators and surgical masks. Products, vendors, or commercial services mentioned or pictured in this article are for illustrative purposes only and are not meant to be endorsements.
Upcoming Events
Save the Date: April 28th, 2020, Tuesday, 7:30am - 5:00 pm. 3rd Arizona School IPM Conference. Online, information to follow.
Check out upcoming Integrated Pest Management Webinars at https://www.epa.gov/managing-pests-schools/upcoming-integrated-pest-management-webinars
For more information about the EPA Schools program: http://www.epa.gov/schools/.
To view all our previous newsletters, visit: https://acis.cals.arizona.edu/community-ipm/home-and-school-ipm-newsletters
Acknowledgements
Thanks to Dr. Peter Ellsworth (University of Arizona, Department of Entomology) for critical review of this newsletter.
This material is based upon work that is supported in part by the National Institute of Food and Agriculture, U.S. Department of Agriculture (USDA NIFA) under the Crop Protection and Pest Management, Extension Implementation Program, award number 2017-70006-27145 which provides Extension IPM funding to the University of Arizona. Information regarding this document is within the guidelines of the Border 2020 Program funded by the U.S. Environmental Agency (EPA) and administered by NADB. Additional support is provided by the University of Arizona – Arizona Pest Management Center (APMC). Any findings, recommendations, services, or organizations that are mentioned, shown, or indirectly implied in this publication do not imply endorsement by the University of Arizona, the USDA or EPA.
